ADSORPTION TO KERATIN SURFACES phobically driven adsorption process by structurally modifying the adsorbing species, and it lends support to the charge to hydrophobic continuum mechanism for the adsorption of conditioning agents to human hair. CHANGES IN THE ADSORPTION MECHANISM THROUGH PH VARIATION Four decades ago Vickerstaff (10) described the attachment of Carbolan dyes (anionic dyes see structure 5) to wool fiber at acid pH as occurring, "with the negatively charged hydrophilic sulfonic group on the positively charged fiber surface and the hydrophobic tail projecting outwards," thus creating a hydrophobic fiber. However, by changing the pH to neutral, he argued that the mechanism of dyeing changes and "will lead to adsorption with the hydrophobic part of the dye on the fiber surface and the hydrophilic sulfonic acid group projecting outwards," thus creating a hydrophilic fiber. o H II OH N- C- CH 3 C12H15-•N=N• SO3H SO3H (5) A Carbolan dye Therefore, more than forty years ago, Vickerstaff suggested that the dye orientation on the fiber surface for these anionic sulfonate dyes could be changed from hydrophilic to hydrophobic bonding by controlling the pH of the dyeing medium, a proposal consis- tent with the charge to hydrophilic continuum hypothesis. Vickerstaff's proof involved chopping the fibers into fine fragments and shaking these in a mixture of benzene and water. In this experiment, the fiber fragments that had been dyed in acid tended to collect in the benzene phase. However, those fiber fragments dyed at neutral pH tended to collect in the aqueous phase. N =N-• SO3Na (6) Orange II dye Since Vickerstaff's experiments involved finely chopped wool fiber fragments, questions about bonding to the internal fiber vs adsorption to the cuticle remained unanswered. Therefore, to test this hypothesis of the ability to change from a hydrophilic to a hydrophobic adsorption process with anionic dyes, we decided to examine a similar system, using Orange II dye (see structure 6) and to test the wettability of the keratin surface by observing the time required for droplets of water to wet out wool fabric that
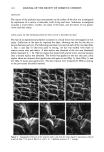
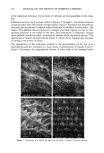
92 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS had been dyed with Orange II at pH 1 (hydrochloric acid) and at pH 7 (phosphate buffer) and then dried overnight (see Figure 2 and Table V). Figure 2 illustrates the droplets of water on three pieces of treated wool fabric after 60-second dyeing, rinsing, and drying overnight. The wool fabric dyed at pH 1 is clearly more hydrophobic than the wool fabric dyed at neutral pH. It is also more hydrophobic than the control fabric washed with 10% sodium lauryl sulfate and rinsed with water and then dried. The data of Table V summarize numerically the effects illustrated in Figure 2, and it demonstrates that wool fabric treated with an anionic dye (Orange II) at acid pH does make the fiber surface less wettable by water than does the same wool dyed at neutral pH. Furthermore, dyeing at a higher dye concentration (5% vs 0.5%) at neutral pH not only causes more dye binding (darker orange), but it also makes the fabric even more wettable. These findings suggest that the adsorption of anionic dyes and presumable anionic surfactants to keratins at acid pH (pH 1) is largely a charge-driven process. Thus the dye binds to the fibers primarily through electrostatic forces, and therefore, the hydrophobic portion of the dye projects outward, creating a hydrophobic hair fiber surface. However, as one increases the pH to neutral, the mechanism of action changes, and it becomes a hydrophobically driven process. As a result, the hydrophobic portion of the dye binds to the fibers, and the hydrophilic part of the dye projects outward, thus creating a more hydrophilic fiber. ORANGE-II {- ORANGE-II pH '•_1_ [0.5%] pH 77' [5.0O/o] pH ?_ •_0.5 •/o'• Figure 2. Illustration of the difference in wettability of keratin fibers after treatment of wool swatches with Orange II dye at acid and neutral pH.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)