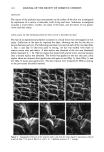
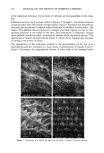
114 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (Figure 3a) were removed at q- 30 minutes (Figure 3b). No significant changes occurred with further replication. The replication of a rough, flaky area on the sole of the foot at 0 and q- 30 minutes is shown in Figure 4. No loss of detail was evident at either low (x 50) or higher (x 500) magnification. Scales were found to remain intact with little or no disruption. The suitability of the method for the replication of fine, delicate structures is demon- strated in Figures 5 and 6. Figure 5 illustrates several hair follicles with intact hairs from the forearm region. The replication method provided details of the cuticular scales on the hair shaft. The topographical details of the sheath, which is wrapped around the base of the hair, as well as of sloughing scales, are also revealed. Figure 5b demonstrates the resolution obtainable using this replication technique, as the spacing between the cuticles is approximately 5 to 10 mm. Figure 6 shows the replication of intricate, fragile structures present on the surface of the leaves of two plants: velvet leaf (Figures 6a, 6b) and coleus (Figures 6c, 6d). Details were excellently reproduced, illustrating the utility •$•IPL ' , 'LE • SOLE.. ß -. ., ß Figure 4. Replication of the sole of the foot: (a) and (b) 0 minutes (c) and (d) + 30 minutes.
REPLICATION OF SKIN 115 ß .• .,. • .,.,• .... ß .... 880311 10K¾ XS00 GOum • :•'L REPLICA -4 , Figure 5. Replicated hair follicles present on the skin. H = hair shaft S = sheath at base of hair shaft C = cuticle. 888867 10KV Figure 6. Replication of the surface of plant leaves: (a) and (b) velvet leaf (c) and (d) coleus. G = globular structures.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)