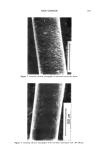
176 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS over the range 0.005% to 0.05% (n = 4). The equation of the line obtained was y = (1.64 X 107 --+ 7.5 X 105)x + (60700 - 11600). This corresponds to a working range of 0.10% to 1.0% for a 20-fold dilution of the formulation. Typical levels are 0.10% to 0.30%. A typical electropherogram obtained for 0.02% diazolidinyl urea, prepared as above, is shown in Figure 1. Elution time is =4 minutes. RESOLUTION OF DIAZOLIDINYL UREA FROM SAMPLE COMPONENTS The method provides excellent resolution of diazolidinyl urea from other detectable sample components in a wide variety of formulations. Figure 2 shows an electrophero- gram of 5 % commercial pain-relieving gel. Diazolidinyl urea is effectively resolved from all other components, including allantoin, and elutes at ca. 4 minutes. Figure 3 shows the resolution of diazolidinyl urea in the shampoo (10%) from the other detected components, some of which are neutrals and elute with the electroosmotic flow (=3 minutes) and another intense peak at ca. 5 minutes (possibly methyl paraben). In Figure 4, the method is successfully demonstrated for a commercial vitamin E/lan- olin-containing hand lotion (5%). Again, diazolidinyl urea is completely resolved from the other components. In this case, two later-eluting species appear, possibly methyl and propyl paraben, although they have limited solubility in water. We have found under these conditions, using standard methanol solutions of the parabens, that they elute at these retention times and that propyl paraben elutes first. The parabens are ionized at elevated pH levels. RECOVERY OF SPIKED SAMPLES Samples which contain diazolidinyl urea as a preservative were spiked with known amounts upon dilution and prior to mixing. Recoveries compared with standards of equal concentration were generally acceptable, and the diazolidinyl urea level was de- termined in some formulations by external standards (i. e., calibration curve). Recoveries are summarized in Table I. To investigate the accuracy of the method a mock hand cream formulation was prepared. It was spiked with 0.2% diazolidinyl urea, and prepared and analyzed in the manner of the preservative-containing formulations. The diazolidinyl urea content was determined (by standard additions) for three separate samplings to be 0.22%, 0.22%, and 0.27%. Recovery was also evaluated by spiking samples after mixing and shaking. The hand lotion was prepared (5%) and mixed as described. The sample was then spiked with Figure 1. Typical electropherogram obtained for 0.02% diazolidinyl urea. Conditions given in text,
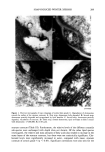
DETERMINATION OF DIAZOLIDINYL UREA 177 Allantoin diazolidinyl urea Figure 2. Electropherogram for pain-relieving gel (5%). Sample preparation and experimental conditions given in text. 0.005% and 0.01% diazolidinyl urea. The recovery was 112% and 92%, respectively. "Daytime" pain-relieving gel was spiked in the same manner, with recoveries of 93% and 88%. METHOD OF STANDARD ADDITIONS Because of the wide variety and complex nature of the formulations containing diazo- lidinyl urea, the method of standard additions was used for analysis. For some products, the matrix effect (i.e., poorer recovery) was greater than for others. In other formula- tions, such as the pain-relieving gel, the amount of diazolidinyl urea determined by
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)