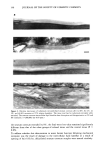
j. Soc. Cosmet. Chem., 46, 141-151 (May/June 1995) Changes in stratum corneum lipid and desmosome structure together with water barrier function during mechanical stress A. V. RAWLINGS, A. WATKINSON, C. R. HARDING, C. ACKERMAN, J. BANKS, J. HOPE, and I. R. SCOTT, Unilever Research, Edgewater, NJ (A.V.R., I.R.S.), and Unilever Research, Sharnbrook, Bedford, UK (A.W., C.R.H., C.A., J.B., J.H.). Received November 20, 1994. Presented in part at the 17th IFSCC International Congress, October 1992, and at the American Academy of Dermatology, December 1992. Synopsis The elastic properties of the stratum corneum have been examined by many investigators, but the mor- phological evidence for changes in the structure of stratum corneum cohesive elements, lipids and desmo- somes, during mechanical extension remains sparse. Additionally, little is known of the effect of mechanical stress on stratum corneum water barrier function. In this study we have examined in detail changes in the structure of the stratum corneum intercellular lipids and desmosomes during linear extension studies on isolated stratum corneum in vitro. Ultrastructural changes have been investigated by electron microscopy, and barrier function has been assessed by measuring water loss through the tissue. Initially, at low extensions of the stratum corneum the structure of the intercellular bilayer lipids appeared normal, during further extension their membrane structure became disrupted, and with continued extension they became progressively disorganized. Desmosomes, by comparison, were more resilient structures, only being per- turbed after large extensions during which intercorneocyte desmosomal links were observed to rupture just before the complete fracture of the tissue. These events were associated with increased water loss through the stratum corneum. If these in vitro events are paralleled in vivo, it appears that stratum corneum lipids are sufficiently fluid to maintain barrier function during small extensions of the skin surface. However, following large extensions the structural changes could lead to perturbed water barrier function, desmosome rupture, and aberrant desquamation, resulting in the appearance of xerotic skin conditions. INTRODUCTION Although stratum corneum extensibility is related to the cohesive properties of the tissue, the relationship between structural elements of the stratum corneum and its elasticity is poorly understood. Intercellular lipids (1) have long been thought to play a role in stratum corneum cell cohesion (for review, see reference 2). However, since the observation that desmosomes are responsible for maintaining the integrity of this tissue subsequent to mechanical rupture (3), it has become increasingly recognized that these protein complexes play a crucial role not only in cohesion of this tissue but that also, 141
142 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ultimately, their breakdown controls the loss of corneocytes from the skin surface (for review, see reference 4). Desmoglein 1 (dsg 1), a desmosomal protein belonging to the cadherin superfamily of adhesion molecules (5), is believed to be the key extracellular component of the desmosome responsible for corneocyte cohesion. Although lectins may also play a role in intercorneocyte cohesion (6), it is likely that the intercellular lipids and desmosomes have the most profound effect on stratum corneum extensibility. The precise relationship between lipids and stratum corneum elasticity, however, re- mains controversial. Several investigators have attempted to address this relationship by comparing the mechanical properties of native and delipidated or solvent-damaged stratum corneum. Middleton et al. (7) reported that diethyl-ether extraction of guinea pig foot pad stratum corneum had no consistent effects on its extensibility, and Park and Baddiel (8) claimed that chloroform extraction of pig corneum did not influence its elastic properties. In contrast, Wildnauer et al. (3) found that diethyl-ether extraction of human stratum corneum increased its breaking strength while only marginally af- fecting its percent elongation. More recently, Leveque et al. (9) demonstrated an increase in the elastic modulus for chloroform-methanol-extracted stratum corneum incubated at 56% relative humidity but not at 73% relative humidity, compared with native cor- neum, thus suggesting that the lipid played a role in plasticizing the stratum corneum. In addition, studies from our laboratory have recently demonstrated that ceramide 1 linoleate influences non-solvent-damaged stratum corneum extensibility behavior, in- creasing flexibility at low humidity (10). Few studies have addressed the potential changes in stratum corneum lipid bilayer structure during stratum corneum extension, mainly because early electron microscopy techniques could not visualize the intercellular lipids (11). In addition, changes in stratum corneum water barrier function during in vitro extension have not been exam- ined despite the fact that the maintenance of stratum corneum flexibility and the proper conformation of the intercellular lipids is essential for an intact barrier. Finally, despite the importance of desmosomal degradation in the desquamatory process, nothing is known as to whether moderate extensions of the stratum corneum may impact upon the structural organization of the desmosome and lead to impaired or delayed proteolysis. Here, we report on our studies to examine the relationship between mechanical stress and the structure and function of the stratum corneum, with emphasis on the role of lipid bilayers and desmosomes. MATERIALS AND METHODS PREPARATION OF STRATUM CORNEUM Stratum corneum was prepared from full-thickness pig skin using standard methods. Briefly, skin hair was shortened using electrical clippers, gently swabbed with cotton wool balls soaked in an aqueous ethanol-chlorohexidine solution (15:75:10v/v Hibi- tane, ICI Pharmaceuticals, UK) to clean the skin surface, and depilated using a com- mercially available depilatory cream, Immac (Whitehall Laboratories, UK) before re- moving 0.2-mm slices of skin with a Davies electric skin dermatome. Stratum corneum was then isolated by trypsin digestion of the viable epidermis (0.25% trypsin in phos- phate-buffered saline, pH 7.4), as described in detail (12). The sheets of stratum
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)