158 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 120.0 80.0 - %T - 40.0 - 0.0 1 4OOO 2 2090 cm -1 2196 cm -1 3000 2000 1400 750 cm-1 Figure 3. Infrared transmittance spectra of deutero lauroyl sarcosine (1) and lauroyl sarcosine (2). 2196 cm- • are due to C - D vibrations. The weak C-H bands in the 3000 cm- • range are mainly due to the few C - H bonds present in the sarcosine moiety of the surfactant. ESTIMATION OF THE SUBSTANTIVITY The substantivity tests with solutions of (d23) lauroyl sarcosinate at desired concentra- tions and pH were conducted as outlined in the experimental section. Infrared bands at 2090 cm- • and 2196 cm- • were observed in all the spectra of the skin after contact with the deuterated surfactant (Figure 4). In order to quantify the amount of adsorbed surfactant, a calibration was needed. The skin-crystal contact is an important parameter in ATR spectroscopy. The intensity of the bands depends on the extent of contact, when all other parameters are kept the same. In order to overcome the variabilities in spectral absorbances due to differences in the skin-crystal contact, a band ratio program was devised. The C-H stretches of skin spectrum were taken to indicate the quality of skin prism contact, and the C - D band absorbances of the sorbed surfactant were normalized to the skin C-H absorbances. For calibrating absorbance measurements, an area of 7 cm x 4 cm was marked on the forearm. A 100ptl aliquot of a solution of N-perdeuterolauroyl sarcosine of known concentration in methanol was spread on the marked area. Effort was made to keep the distribution of the solution uniform over the 28-cm 2 area. After the solvent evaporated, an IR scan of the area was obtained. The application of the standard --1 solution and IR scanning was repeated five times. The ratio of absorbance at 2196 cm --1 (C-D from the deposited deutero lauroyl sarcosine) to the absorbance at 2849 cm (C-H from skin) was plotted versus ptg of standard applied per unit area of the skin. A linear calibration (Figure 5) was obtained. The same band ratio program was applied to the IR spectral data of the surfactant-treated skin, and the calibration was used to calculate the lauroyl sarcosine sorbed (ptg/cm 2) on skin.
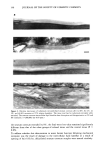
N-LAUROYL SARCOSINATE 15 9 1.5 1.2 l Abs. .6 0.0 4OOO 3000 2000 1500 900 crn-1 Figure 4. Infrared absorbance spectra of human skin before (1) and after (2) contact with sodium deutero lauroyl sarcosinate. The highlighted spectral region between 2000 cm-• and 2200 cm-• indicates the appearance of C-D bands at 2090 cm- • and 2196 cm- • in the skin spectrum after contact with the surfactant. 40 30 20 I•g d23 surfactant Sq.cm. skin 10 0.0 0.2 0.4 0.6 0.8 1.0 Abs. C-D/Abs. C-H Figure 5. Calibration for estimation of surfactant on skin. EFFECT OF pH, CONCENTRATION, AND CONTACT TIME Perdeuterolauroyl sarcosine adsorbs on skin at •g/cm 2 levels. Subsequent water rinses remove the sorbed surfactant. Approximately 50% of the initially sorbed surfactant is removed by four rinses each with 100 ml of water. The precision of the test method was
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)