STRATUM CORNEUM LIPIDS 143 corneum were cut into rectangular strips measuring 1 X 2.5 cm, a piece of plastic tape (Flow tape, Flow Laboratories, UK) was attached to each end of the stratum corneum, and a 6-mm hole was punched into the center of the piece of tape. During this whole procedure, the orientation of the stratum corneum was maintained to ensure that the external surface was known. Preliminary experiments showed that the depilatory treatment followed by the extensive post-washing treatment during stratum comeurn isolation had no effect on stratum corneum extensibility behavior, water barrier function, or the lameliar structure of intercellular lipids as visualized by electron microscopy (data not shown). ELECTRON MICROSCOPY Samples of stratum corneum were fixed for 2 h in 0.1 M sodium cacodylate buffer, pH 7.4, containing either ruthenium tetroxide (0.2%) or osmium tetroxide (1.0%) and potassium ferrocyanide (2.5%). Following fixation, samples were rinsed with buffer dehydrated through a graded series of alcohol to propylene oxide and embedded in TAAB resin (TAAB Lab., Aldermaster, UK). Thin sections were cut using a Reichert- Jung Ultra-microtome and viewed with a JEOL 100 CXII transmission electron micro- scope after staining with lead citrate. IN VITRO EXTENSION OF STRATUM CORNEUM Stratum corneum samples were pre-equilibrated to 97% relative humidity for 1 h by suspension from hooks in humidity chambers containing a saturated solution of potas- sium sulphate at approximately 2 iøC before being extended to approximately 2%, 5%, and 8% of their original length or to their breaking points (approximately 10% exten- sion) at 20 mm/min, using a linear extensometer (Rheometer, Diastron Ltd, UK). Samples were then either maintained at these extensions for 1 h or relaxed overnight prior to water barrier function measurements. Unstretched pieces of stratum corneum, similarly maintained overnight, served as control tissue. For some studies, stratum corneum samples were first delipidated using 20 volumes of chloroform-methanol (2:1) for 4 h before equilibrating to 97% relative humidity and conducting extensibility and water barrier assays. IN VITRO WATER BARRIER MEASUREMENTS OF STRATUM CORNEUM The efficiency of the water barrier in tissue subjected to different extensions was mea- sured using standard techniques (10). Briefly, 500 ptl of distilled water was placed in the wells of water vapor transmission cells, the stratum corneum was placed over the well, supported on a stainless steel frit, and covered with an O-ring, and the top half of the cell was screwed into place so that a watertight seal was achieved. The area of exposed tissue, which is the cross-sectional area of the center of the water vapor transmission cell, was 0.785 cm 2. In order to measure the influence of continued extension on barrier function, the lower half of the cell was placed under the stratum corneum while the tissue was under tension on the extensometer and quickly fixed in place using a small quantity of cyanoacrylate adhesive before assembly of the cell was completed. Initial
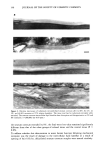
144 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS weight measurements were taken (Wo) , and the cells were placed into a desiccating chamber containing dried silica gel at 37øC. Weights were taken again at approximately 12 h (W•2) and 24 h (W24), and the water vapor transmission rate (WVTR) was calculated from the final 24-h reading. The 12-h measurement was taken to assess linearity of water loss. The WVTR (mg/cm2/h) was calculated as follows: Wo -- W2 4 WVTR = Area x 24 EXTRACTION AND ANALYSIS OF DESMOGLEIN 1 To investigate whether physical stretching of the tissue was associated with increased degradation of desmosomal proteins, pieces of stratum corneum were extended to 8% of their original length, allowed to relax overnight, and then extracted for determination of dsg 1 levels as previously described (13). STATISTICAL ANALYSIS Results are reported as mean + standard deviation. Mean values of WVTR were compared using a Student's t-test. Significance was set at the 5% level. RESULTS CHANGES IN STRATUM CORNEUM LIPID AND DESMOSOME STRUCTURE DURING IN VITRO EXTENSION Under mechanical stress, stratum corneum demonstrated a three-phase stress-strain relationship, as previously reported (3). The initial slope of the stress-strain curve, the Hookean region, was demonstrated up to 4% extension, followed by a small plateau period (4-6% extension) after which the tissue stiffened before breaking (Figure 1). When examined by electron microscopy, using ruthenium tetroxide to visualize the lipid structures, distinct changes in stratum corneum lipid and desmosome structure were associated with these different regions. Normal intercellular lipid lamellae struc- tures were seen in up to 4% extension of the stratum corneum (Figure 2a,b), but beyond this region, at 5% (Figure 2c) and 8% extensions (Figure 2d), the bilayer structure of the lipids was disrupted and disorganized in places, with the suggestion of a more "loosely packed" structure. These structural changes were small at 5% extension, but were far greater at 8% extension. Changes in desmosome morphology were not apparent during limited extension, and their structures were identical to desmosomes present in unstretched tissue (Figure 3a). However, these structures were consistently ruptured when stratum corneum was extended to 8% (Figure 3b,c). The same changes in lipid and desmosome morphology were apparent irrespective of the direction of extension (unpublished results). For tissues subjected to 5% extension, the structure of the intercellular lipid lamellae still appeared marginally disrupted following relaxation, similar to tissue that remained extended and held (Figure 4a). When stratum corneum was subjected to 8% extension and relaxed, lipid lamellae appeared normal, but in some areas of the tissue they were separated by large electron-lucent intercellular spaces (Figure 4b). Under these condi- tions, desmosomes also appeared incapable of reforming their natural structures upon
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)