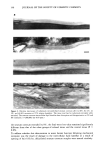
STRATUM CORNEUM LIPIDS 149 Control T.T. 2% Extension 5% Extension 8% Extension * Significantly different to control p0.05 ][] ExtendandandTissueTissue Held I Extended Relaxed Figure 5. Histogram of changes in stratum corneum water barrier function during in vitro extension studies. Note large increases in water vapor transport rate at 8% extension (n = 5 P 0.05). logical implications of these results and have shown that disruption of the organization of the intercellular lipid bilayer structure is associated with perturbations in water barrier function in vitro. These increases in water loss were not due to stretching of the tissue causing distension of hair follicles, as changes in water loss were not observed for solvent-delipidated stratum corneum treated in the same way. These findings empha- size, therefore, that the precise molecular packing of stratum corneum lipids is vital for efficient water barrier function (1,11,14,15) and that this organization is prone to disruption by mechanical stress. When stratum corneum was extended close to the breaking point, the structure of the desmosomes was lost and they appeared physically ruptured. This is clearly different from the fate of desmosomes in normal desquamation where, by electron microscopy, the extracellular, cohesive portion of the desmosome complex appears to be digested internally, initially becoming vacuolated (i.e., a loss of the central core of electron-dense material) before detaching from the corneocyte envelopes. During that process, lipid lamellae appear to flow around the desmosomal remnants, encapsulating and isolating this degrading structure (15,16). We suggest that in vivo the encapsulation of such degraded desmosomal structures by stratum corneum lipids during desquamation is essential for the maintenance of optimal barrier function as well as for decreasing intercellular cohesion and facilitating, thereby, loss of cells from the surface of the skin. Recently, in vitro desquamation models have indicated, first, that the detachment of corneocytes is facilitated by the action of serine proteases (17), in particular chymotryp- sin-like enzymes (18) that appear to be located within the extracellular space (19,20) and degrade the desmosomal cadherin dsg 1 and, second, that the desquamation process is crucially dependent on the water content of the tissue (21). However, our results indicate that mechanical stretching was not associated with increased proteolysis of intact dsg 1, suggesting that the changes in the desmosomes seen in the current studies were a direct result of mechanical fracture. Desmosomes that had been mechanically torn apart under high extensions failed to
150 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS correctly anneal and reform their original ultrastructure upon relaxation. The inability to isolate or repair these disrupted structures was associated with an increased WVTR. Thus the combination of desmosomal rupture and changes in the architecture of the stratum corneum lipids probably contributes to the increased water loss observed in vitro. We consider it likely that similar perturbations to stratum corneum water barrier function occur in vivo during mechanical extension, particularly under conditions of low environmental humidity on exposed body sites that undergo repeated flexures, e.g., the knuckle region of the hands and elbows. The resulting structural abnormalities, through interfering with the normal process of desquamation, may lead to the formation of skin xerosis, or increase its severity. Furthermore, these changes are likely to be more pronounced in winter, when the marked seasonal decrease in intercellular lipid levels (22) and lipid types (23) may weaken barrier resilience. In fact, we have shown that these structural changes occur at lower extensions for stratum corneum incubated at low humidity compared with tissue incubated at high humidity. Typically, changes in lipid membrane structures were seen at less than 4% extension, and the tissue ruptured at 6% extension (24). Thus, in vivo, in the outermost layers of the stratum corneum, which is exposed to low environmental humidities and has less endogenous natural moisturizing factor, these structural changes may occur at much lower extensions than we have reported here. Aberrations in the structure of the stratum corneum lipids in the superficial stratum corneum are known to be associated with the appearance of xerosis, which is probably due to changes in desquamatory enzyme activities (16). As the desquamatory proteases are known to be localized within the intercellular lipids (19,20), the physical organi- zation and properties of the stratum corneum lipids will influence their activities. The structural changes in barrier lipids seen in the present study, therefore, are likely to have adverse effects on their activities. In addition, changes in stratum corneum water content, as a result of perturbed barrier function, could adversely influence the activity of enzymes involved in other events crucial to the normal maturation of the stratum corneum (for review, see reference 4). Finally, inferior barrier properties will allow greater penetration of harmful irritants through the stratum corneum into the under- lying epidermis, leading to inflammation and disturbances in the differentiation path- way and ultimately producing a stratum corneum of inferior quality, more prone to further mechanical disruption. In conclusion, during mechanical extension of stratum corneum a loss of lipid organi- zation and desmosomal structure occurs with concomitant disturbance of barrier func- tion. Incorrect desmosomal reannealment and encapsulation with intercellular lipids, together with the inability to correctly maintain normal multiple intercellular lamellae ultrastructure, could account for this defect in barrier function. The loss of structural and spacial organization of lipids and desmosomes between the corneocytes will have an adverse effect on stratum corneum barrier function in vivo and on the in vivo functionality of proteolytic enzymes, particularly those responsible for desmosomal digestion. These events will precipitate the development of skin xerosis or worsen an existing xerotic condition. ACKNOWLEDGMENTS We would like to thank Dr. A. I. Magee, National Institute for Medical Research, Mill Hill, England, for providing the antibody to Dsg 1.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)