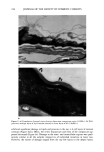
254 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 1.6 C12 Alkyl Sulfate •_• 1.2 C14 Alkyl Sulfate • Sulfate • 0.8 • 0.4 C 8 Alkyl Sulfate ! I 0 0.01 0.02 0.03 0.04 0.05 CONCENTRATION {MOLES\LITER) Figure 1. Adsorption isotherms for alkyl sulfates with isolated callus powder in vitro. Experimental con- ditions: callus, 48 to 80 mesh, pH 7.0, 6-h incubation at 40øC. Data taken from ref. 2. Thus the apparent saturation at the CMC may not be attributed to saturation of binding sites but rather to the availability of a fixed dose of monomer above the CMC. These data are also consistent with the hypothesis that the surfactant monomer rather than the micelie is the species that interacts with the skin. Thus the saturation phenomenon observed in the isotherm may be related to this particular solution property of the surfactants, i.e., their propensity to micellize above a critical concentration. One can then speculate that the greater the surfactant binding to keratin, the greater the damage to the membrane once this occurs extensively the surfactant will penetrate through the stratum corneum into the living tissue. In the living tissue, surfactant denaturing activity continues, but this time an inflammatory reaction sets in to repair the damage. What does greater surfactant binding mean? It could mean that the higher concentrations of surfactant monomer available to act on the stratum corneum will produce more noticeable end effects. It could mean also the type of binding that results from particular structural aspects of surfactants. This leads to end effects such as hy- drophobic or ionic interactions and the consequences of these interactions, which could include denaturation, swelling, decreased intercorneal cohesion, or alterations in the lipid part of the membrane. These aspects will be explored further. Faucher and Goddard (3) have studied the binding expressed as sorption of detergents to keratinaceous substrates. They reported that SLS sorption increased over time. It followed a linear dependence on the square root of time, consistent with a diffusion process. The slopes of the uptake vs square root of time lines can be regarded as a measure
SURFACTANTS AND STRATUM CORNEUM 255 of rate of sorption. Again the rate function for surfactant uptake vs concentration at one hour is biphasic and intersects at the CMC of SLS. (Figure 2). The CMC of SLS is between 7 and 10 raM, depending on the conditions in various studies. Faucher and Goddard (3) also examined binding of sodium lauryl ether sulfates con- taining different levels of ethoxylation to bleached human hair, a keratinaceous substrate similar to stratum corneum (Figure 3). They found that increasing the degree of ethox- ylation concomitantly decreased binding. Various explanations are possible for this phenomenon. As ethoxylation increases, the molecular size increases, making it more difficult to penetrate the keratin matrix. Additionally, the CMC decreases, and thus the surfactant monomer level decreases, rendering less surfactant available to interact with the substrate, assuming the monomer is the interactive species. The relationship of CMC to skin reactivity of surfactants will be addressed in detail in a later section. More recent studies of Ananthapadmanabhan et al. (4) examined binding of surfactants to stratum comeurn in greater detail. They looked at a broad concentration range, along with time, temperature, and pH effects. Figure 4 shows binding of SLS and cocoyl isethionate at doses up to 80 mM, and the findings again confirm a change of slope at the CMC. Like the results of Faucher and Goddard, binding continues to increase but at a slower rate above the CMC. Thus while monomer concentration is important to binding, it does not totally dictate the binding parameters, a phenomenon that will be discussed later. Not unexpectedly, binding also increases with increasing temperature. The relationship of binding to time of exposure was surprising (see Figures 4 and 5) (data 60 ul 50 o • 40 ß .-, 30 • 20 lO Stratum Corneum Bleached Hair o o 1 2 3 4 5 6 7 8 9 lO CONCENTRATION OF SIS IN PERCENT Figure 2. Concentration dependence of sorption rate: uptake of sodium lauryl sulfate by stratum comeurn and bleached hair. Absorption of SLS is measured as percent increase over the weight of the dried, rinsed substrate. Data taken from ref. 3.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)