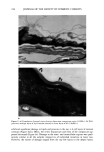
270 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ANIONIC SURFACTANT BINDING AT pH 9 H2N COO' 0.80- • S03 '00C NH• ANIONIC SURFACTANT BINDING AT pH 3 Keratin Fiber OSO3' Anionic Surfactant Van der Waals Attraction Electrostatic Repulsion Ionic Attraction Figure 10a. Schematic depicting possible binding sites for anionic surfactants to keratin at pH 9 and pH 3. Note that for anionic surfactants more potential binding sites exist at pH 3, where both ionic (to positive charges on the keratin) and hydrophobic (Van der Waals) binding opportunities occur. At pH 9, for anionics most of the binding is probably hydrophobic. Importantly, note that hydrophobic binding of the tails of the charged surfactant to sites on the keratin results in electrostatic repulsion between surfactant head groups and hence unfolding of the keratin chain. Ionic binding to sites on the keratin results in Van der Waals attraction between the hydrophobic tails of the surfactants, leading to shrinkage or tightening of the keratin chain. and arginine, leaving them positively charged. Thus, in addition to the hydrophobic bonding, there would also be considerable ionic bonding between the positively charged amino acids and the anionic surfactant. As illustrated in the schematic, when this happens, the hydrophobic tails associate via Van der Waals forces and may cause shrink- age of the membrane. But the other type of bonding, hydrophobic bonding of surfactant, still occurs to the various hydrophobic parts of the protein, and this is countering the shrinkage due to repulsive forces between the negatively charged surfactant head groups, hence the "bulge". For cationic surfactant interaction with keratin at pH 9 (Figure 10b), the main type of
SURFACTANTS AND STRATUM CORNEUM 271 CATIONIC SURFACTANT BINDING AT pH 9 '00C H2N ' (•'•3)3 CO0-, N(CH3)3'"'v•' CO0' N(CH3)3-,,• CH 3 + ' + ' C• + ' •,,,•._..N CH•)• CO0. A• NH2 COO' CATIONIC SURFACTANT BINDING AT pH 3 H3N+ COOH NH2 ""' 'v'm, • N(•H3)3 + N(CH3)3 + HOOC NH3 + Keratin Fiber N(CH3)3 + Cationic Surfactant Van der Waals Attraction Electrostatic Repulsion Ionic Attraction Figure 10b. Schematic depicting possible binding sites for cationic surfactants to keratin at pH 9 and at pH 3. The opposite effects exist for cationic surfactants (as opposed to anionic surfactants), where most of the ionic binding occurs at pH 9 to negative charges on the keratin along with the hydrophobic (Van der Waals) binding of the tails to the keratin. binding would likely be ionic, as the positively charged surfactant would bind to the negatively charged sites on the keratin, which are due to the glutamic and aspattic acid anion. This causes shrinkage of the membrane due to association of the hydrophobic tails of the cationic. Some hydrophobic binding occurs to those sites present on the keratin (the "bulge"), but the net result is shrinkage. At pH 3, on the other hand, the major type of binding is hydrophobic, and the dangling cationic head groups exhibit electrostatic repulsive forces. Thus, greater swelling occurs at acid pH for cationic surfactant-protein interactions, but the net effect appears to be shrinkage rather than swelling at the acid pH for cationics (20). Absolute binding results also support these potential interactions. Greater amounts of anionic surfactants are reported to bind to stratum corneum keratin at the lower pns (43). This makes sense because, at the acid pH, several types of potential binding sites are available on the protein that can interact with the surfactant, i.e., both ionic and hydrophobic binding sites (Figure 10a). At the alkaline pH, more or less only hydro-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)