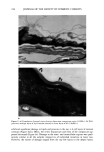
SURFACTANTS AND STRATUM CORNEUM 271 CATIONIC SURFACTANT BINDING AT pH 9 '00C H2N ' (•'•3)3 CO0-, N(CH3)3'"'v•' CO0' N(CH3)3-,,• CH 3 + ' + ' C• + ' •,,,•._..N CH•)• CO0. A• NH2 COO' CATIONIC SURFACTANT BINDING AT pH 3 H3N+ COOH NH2 ""' 'v'm, • N(•H3)3 + N(CH3)3 + HOOC NH3 + Keratin Fiber N(CH3)3 + Cationic Surfactant Van der Waals Attraction Electrostatic Repulsion Ionic Attraction Figure 10b. Schematic depicting possible binding sites for cationic surfactants to keratin at pH 9 and at pH 3. The opposite effects exist for cationic surfactants (as opposed to anionic surfactants), where most of the ionic binding occurs at pH 9 to negative charges on the keratin along with the hydrophobic (Van der Waals) binding of the tails to the keratin. binding would likely be ionic, as the positively charged surfactant would bind to the negatively charged sites on the keratin, which are due to the glutamic and aspattic acid anion. This causes shrinkage of the membrane due to association of the hydrophobic tails of the cationic. Some hydrophobic binding occurs to those sites present on the keratin (the "bulge"), but the net result is shrinkage. At pH 3, on the other hand, the major type of binding is hydrophobic, and the dangling cationic head groups exhibit electrostatic repulsive forces. Thus, greater swelling occurs at acid pH for cationic surfactant-protein interactions, but the net effect appears to be shrinkage rather than swelling at the acid pH for cationics (20). Absolute binding results also support these potential interactions. Greater amounts of anionic surfactants are reported to bind to stratum corneum keratin at the lower pns (43). This makes sense because, at the acid pH, several types of potential binding sites are available on the protein that can interact with the surfactant, i.e., both ionic and hydrophobic binding sites (Figure 10a). At the alkaline pH, more or less only hydro-
272 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS phobic binding sites are available to interact with the surfactant, resulting in less surfactant binding. The issue in deliberation is the relationship between binding and irritation potential, that is, which type of binding is most detrimental to the skin and why. Greater swelling and reversible denaturation of the protein occurs in the presence of an anionic surfactant at higher alkaline pH due to the hydrophobic interactions with anionic surfactants and resulting electrostatic repulsion at the higher pH, even though there is overall less surfactant binding due to the absence of ionic binding. We don't know whether or not this translates into higher levels of irritation. These studies have yet to be done. For cationic surfactants, e.g., cetyl trimethyl ammonium chloride, we know they can be quite irritating (refs. 22, 44, 45 Table I), i.e., they are comparable to SLS but do not swell stratum corneum to a significant extent at neutral and alkaline pHs. This absence of swelling is likely due to the fact that for cationic surfactants, ionic bonding is prominent at the alkaline pH, where negatively charged aspartat glutamate will bind to the cationic surfactant and the hydrophobic surfactant tails associate and can actually shrink the membrane (see Figure 10b). Absolute binding results also show that cationic surfactants bind stratum corneum to a greater extent, especially at alkaline pH (44), even though they induce very little swelling at this pH. This raises the question of the importance of swelling and the type of binding (hydrophobic or ionic) and its conse- quential effects on the membrane to the irritation mechanism. More studies will be needed to determine which parameters are most important. Such studies are difficult to interpret because of confounding factors such as micellization and surface activity. The answers to the puzzling dilemma await the intellect and creativity of future surfactant scientists. REFERENCES (1) P.J. Namdi, M. E. Grant, and D. R. Robinson, Destabilization of collagen structure by amides and detergents in solution, Int. J. Peptide Res., 25,206-212 (1985). (2) G. Imokawa and J. Mishima, Cumulative effect of surfactants on cutaneous horny layers: Adsorption onto human keratin layers in vivo, Contact Dermatitis, 5, 357 (1979). (3) J. A. Faucher and E. D. Goddard, Interaction of keratinaceous substrates with sodium lauryl sulfate: 1 Sorption, J. Soc Cosmet. Chem., 29, 323-337 (1978). (4) K. P. Ananthapadmanabhan, K. K. Yu, C. L. Mayers, and M.P. Aronson, Binding of surfactants to stratum corneum, J. Soc. Cosmet. Chem., 47, 185-200 (1996). (5) L. D. Rhein, "In Vitro Interactions: Biochemical and Biophysical Effects of Surfactants on Skin," in S•rfactants i, Cosmetics, 2nd ed., Chapter 18, Surfactant Science Series, M. Rieger and L. Rhein, Eds. (Marcel Dekker, New York, 1997), Vol. 68, pp. 397-426. (6) P. D. Wertz, D.C. Swartzendruber, D. J. Kitko, C. Kathri, M.D. Madison, and T. Downing, The role of corneocyte lipid envelope in cohesion of the stratum corneum, J. Invest. DermatoL, 89, 169-172 (1987). (7) D.C. Swartzendruber, P. W. Wertz, M.D. Madison, and D. T. Downing, Evidence that the corneo- cyte has a chemically bound lipid envelope, J. Invest. DermatoL, 88, 709-713 (1987). (8) C.L. Froebe, F.A. Simion, L.D. Rhein, R.H. Cagan, and A. Kligman, Stratum corneum lipid removal by surfactants: Relation to in vivo irritation, Dermatologica, 181, 277-283 (1990). (9) M. Rieger, Human epidermal responses to sodium lauryl sulfate exposure, Cosmet. Toilerr., 109, 6-74 (1994). (10) A.W. Fulmer and G.J. Karmer, Stratum corneum lipid abnormalities in surfactant-induced dry, scaly, skin,J. Invest. DermatoL, 36, 598-602 (1986). (11) G. Imokawa, S. A. Kasaki, Y. Minematsu, and M. Kawai, Importance of intercellular lipids in water
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)