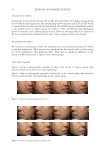
5 COMPARATIVE CLINICAL EVALUATION OF C LAPPACEA formulated using C lappacea. The effects of the new cream on skin lightening, firmness, and elasticity were evaluated using noninvasive measurements. MATERIALS AND METHODS RESEARCH DESIGN This randomized, double-blind, 5 week, pre- and post-comparison clinical trial was conducted from September – October 2017. This study was conducted in compliance with the ethical principles of the Institutional Review Board (IRB) and Institutional Ethics Committee of the Institute of Dermatology, Bangkok, Thailand. The study design was reviewed by the appropriate ethics committees and approved for clinical implementation (Study Code IRB/IEB 002/2561). All research was conducted according to the ethical principles of the Helsinki Declaration (1964). This study included 44 Asian females ages 30–55 with at least 1 facial melasma site. The subjects were fully informed about the purpose of the study and provided written consent. Exclusion criteria included (1) pregnant or breastfeeding, (2) taking oral contraceptives or hormone treatment, (3) a history of cancer or skin cancer, (4) use of topical anti-aging treatment (including anti-aging products, whitening substances, bleaching agents, topical retinoids, and alpha- or beta-hydroxy acid) within 2 months preceding the screening visit, and (5) treatment with aesthetic lasers, Botox, filler, or thread lift within 6 months preceding the screening visit. Subjects with other facial conditions that could potentially affect the study results were also excluded. Subjects with a history of allergic reactions to cosmetic products, sunscreens, or ingredients in the tested product were not recruited for the study. TEST PRODUCT Subjects were randomly assigned to apply either C lappacea cream or a control (placebo) cream to their entire face twice daily for 4 weeks. The test (labeled A) and placebo (labeled B) creams were packaged in identical 2 g tubes, for twice daily use. The test product was composed of C lappacea extract (0.1%) with other ingredients (Appendix) and broad- spectrum ultraviolet A and ultraviolet B filters ethylhexyl methoxycinnamate, octocrylene, butyl methoxydibenzoylmethane, and titanium dioxide were included to provide sun protection at SPF 30. The control product was identical but excluded the C lappacea extract. PRODUCT APPLICATION Subjects were instructed to apply the test or control cream to the entire face twice daily (morning and bedtime) for 4 weeks. After washing the face with gentle liquid soap (provided), subjects gently squeezed ½ tube of cream (approximately 1 g) onto the back of their hand, which they then distributed across their face in five dots (forehead, nose, two cheeks, and chin) using a fingertip. On the forehead, nose, and chin, subjects gently massaged upward until the product penetrated the skin. On cheeks, subjects gently massaged in a circular direction, from the lower cheek to the upper cheek, until the product penetrated the skin. Sunscreen (provided) was thoroughly applied on top of the cream. All subjects were asked
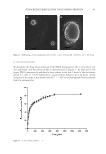
6 JOURNAL OF COSMETIC SCIENCE to use only the mild facial soap and sunscreen supplied by the researchers. No other dermo- pharmaceutical or cosmetic products were authorized for facial use during the study. The first 14 tubes of product were provided to the subjects at their first clinic visit and the remaining 14 tubes were provided at the second visit. Because normal conditions of use required applying the cream at home, researchers could not perform compliance control. Subjects were instead asked to complete a daily log and note the number of applications. Empty tubes and completed daily logs were returned to the researchers at follow-up visits for compliance assessment. STUDY PROCEDURE At the beginning of the experiment and again after 2, 4, and 5 weeks, subjects were interviewed and their faces were examined, photographed by VISIA® (Canfield Scientific, Inc., Parsippany-Troy Hills, New Jersey), and instrumentally measured for efficacy assessment. Skin phototypes, subject medical history, and current medications were also recorded. A dermatologist monitored subjects for any side effects during each clinical visit. Subjects completed a subjective questionnaire at the end of the study. The questionnaire used a four-point scale (strongly disagree, disagree, agree, and strongly agree) to assess each subject’s perspective on the products’ organoleptic characteristics and global efficacy. Subjects were also asked if they would continue to use this product in the future. EFFICACY ASSESSMENTS To obtain quantitative measurements of skin quality, subjects attended the laboratory without applying any product to the face since the previous evening (except the morning wash). They were acclimated in a climate-controlled room at 20–22°C and 50% ± 2% relative humidity for at least 20 min before any skin measurements were taken. After the acclimation period, two measurement points, one melasma, and one adjacent site, were selected and mapped on a plastic sheet. The midpoint between Targus and the lateral mouth angle on the left cheek was also mapped for skin biomechanical measurement. Small holes were cut at the measurement points on the plastic sheet to create openings for the measurement probes. This skin mapping process was used as a guide to ensure measurement sites were the same across all subjects and clinic visits. Melanin levels at each site were determined using a narrowband spectrophotometer (Mexameter® MX18, Courage + Khazaka Electronic GMBH, Köln, Germany). A 5 mm probe emitted specific light wavelengths and calculated the amount of light absorbed by the skin as the melanin index (MI) and erythema index. An assigned medical technician performed the measurement twice in each area. Only the MI, which represents the melanin content of the skin, was analyzed for this study. A decrease in MI signified improvement of pigmentation. Measurements are expressed in arbitrary units (AU). Skin biomechanical properties were measured using a Cutometer® (MPA580, Courage + Khazaka Electronic GMBH, Köln, Germany). The Cutometer® (Courage + Khazaka Electronic GMBH, Köln, Germany) uses a probe to apply negative pressure to the skin for a defined period and then records the degree of skin deformation into the probe. In this study, a 2 mm-diameter probe was used at a constant vacuum pressure of 500 mbar,
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)