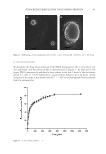
45 SUNSCREEN FORMULATION CONTAINING PROPOLIS antimicrobial, antioxidant, and anti-inflammatory properties of the compound (18,20). In this study, the photoprotective properties of PLGA nanoparticles encapsulating PFs were investigated. The nanoparticles were characterized physiochemically and the release profile of PFs from nanoparticles was determined. Finally, the efficacy of the formulations in exerting sun-protecting effects was evaluated. Preliminary studies showed that higher PLGA concentration as well as longer homogenization time could increase the size and PdI of the nanoparticles (35,37). The findings were in accordance with Iadnut et al. (27), which developed propolis-containing PLGA nanoparticles to assess the antifungal activity against Candida albicans. The observed increase in particle size at higher PLGA concentrations may probably be due to increasing viscosity and therefore subsequent poor dispersibility of PLGA into aqueous phase (38). As represented in Figure 3, the in vitro release study revealed a biphasic pattern containing an initial burst release of PFs from the nanoparticles, which was due to release of flavonoids that were accumulated in the surface of particles, followed by a sustained and prolonged release profile in further time intervals, which belonged to the slow diffusion rate of flavonoid molecules that were entrapped into the nanoparticles. Similarly, Derman et al. (39) showed a slow release of caffeic acid phenolic esters, which were encapsulated in the PLGA nanoparticles. In the literature, the sun protection effects of ethanolic propolis extracts have been proven (3,4,17). In this study, determination of SPF using in vitro method revealed higher SPF values of propolis-containing nanoparticles compared to free PFs. It is also suggested that PFs have the potency to ameliorate the UV-induced skin erythema by exerting anti- inflammatory, antioxidant, and ROS scavenging properties (11,12). In agreement with the data, Sahlan et al. (18) showed that encapsulation of propolis in casein micelles could improve the photoprotection activity by increasing the SPF value. Although the sunscreen formulation containing plain nanoparticles exhibited some degree of photoprotection (i.e., the calculated SPF value of 6.4 ± 0.78), but the calculated SPF value was much lower than the appropriate values in free propolis and propolis-containing nanoparticles. The slight photoprotection of the plain nanoparticles can be justified by their opalescent characteristics (40). CONCLUSION In this study, the photoprotection effect of PF-loaded PLGA nanoparticles was investigated, and it was shown that entrapment of PFs into the PLGA nanoparticles can improve their SPF. However, it is interesting to investigate the effects of the prepared nanoparticles on reduction of skin inflammatory biochemical markers such as enzymatic activities of COX-1, COX-2, 5-lipooxygenase, and myeloperoxidase, as well as secretion of PGE2 and TNF-α. Moreover, the effects of the nanoparticles on expression levels of antioxidant enzymes such as glutathione S-transferases A1-2 (GSTA1-2), GSTA3, and GSTA4, and the determination of the ROS activity in skin are yet to be studied. Investigation of the cellular uptake of nanoparticles in skin layer using confocal laser microscopy and determination of the uptake mechanism is also worthy of investigation. Finally, the efficacy of nanoparticles should be determined following clinical studies on human skin.
46 JOURNAL OF COSMETIC SCIENCE ACKNOWLEDGMENTS This study was designated as the dissertation of Asa Chavoshi (PharmD candidate, School of Pharmacy, Hamadan University of Medical Sciences) and was made possible by financial supports received from deputy of research and technology, Hamadan University of Medical Sciences, Hamadan, Iran under a grant [No. 9611247561]. The authors should thank Dr. Bahareh Afra (Pharm.D, School of Pharmacy, Hamadan University of Medical Sciences) for her efforts in performing experimental sections. REFERENCES (1) J. M. Sforcin, Biological properties and therapeutic applications of propolis, Phytother. Res, 30, 894–905 (2016). (2) M. M. Endo, C. R. A Estrela, A. H. G Alencar, D. A. Decurcio, J. Almeida Silva, and C. Estrela, Antibacterial action of red and green propolis extract in infected root canal. Revista Odonto Ciência, 32, 99–103 (2017). (3) D. M. Lopes and S. B. McMahon, Ultraviolet radiation on the skin: a painful experience?, CNS Neurosci. Ther., 22, 118–126 (2016). (4) W. A. D. S Almeida, A. D. S. Antunes, R. G. Penido, H. S. D. G. Correa, A. M. D. Nascimento, A. L. Andrade, V. R. Santos, T. Cazati, T. R. Amparo, C. H. B. de Souza, K. M. Freitas, O. D. H. dos Santos, L. R. D. Sousa, and V. M. R. dos Santos, Photoprotective activity and increase of SPF in sunscreen formulation using lyophilized red propolis extracts from Alagoas, Revista Brasileira de Farmacognosia, 29, 373–380 (2019). (5) B. Yavari, R. Mahjub, M. Saidijam, M. Raigani, and M. Soleimani, The potential use of peptides in cancer treatment, Curr. Protein Pept. Sci., 19, 759–770 (2018). (6) A. Stellavato, A. V. A. Pirozzi, S. Donato, I. Scognamiglo, S. Reale, A. Di Pardo, S. Filosa, V. Vassalo, G. Bellia, M. De Rosa, and C. Schiraldi, Positive effects against uv-a induced damage and oxidative stress on an in vitro cell model using a hyaluronic acid based formulation containing amino acids, vitamins, and minerals, BioMed. Res Int., 2018 Article ID 8481243, 11 Pages (2018). (7) M. K. Montes de Oca, R. Pearlman, S. F. McClees, R. Strickland, and F. Afaq, Phytochemicals for the prevention of photocarcinogenesis, Photochem., 93, 956–974 (2017). (8) S. Tampucci, S. Burgalassi, P. Chetoni, and D. Monti, Cutaneous permeation and penetration of sunscreens: formulation strategies and in vitro methods, Cosmetics, 5, 1–17, (2018). (9) K. N. Jallad, Chemical characterization of sunscreens composition and its related potential adverse health effects, J. Cosmet. Dermato.l, 16, 353–357 (2017). (10) M. Ghazipur, R. McGowan, A. Arslan, and T. Hossain, Exposure to benzophenone-3 and reproductive toxicity: a systematic review of human and animal studies, Reprod. Toxicol., 73, 175–183 (2017). (11) P. D. Majhi, A. Sharma, A. L. Roberts, E. Daniele, A. R. Majewski, L. M. Chuong, A. L. Black, L. N., Vandenberg, S. S. Schnider, K. A. Dunphy, and D. J. Jerry, Effects of benzophenone-3 and propylparaben on estrogen receptor–dependent r-loops and DNA damage in breast epithelial cells and mice, Environ. Health. Perspect., 128, 017002 (2020). (12) V. Romero, L. O. Guerra, L. Aeillo, and G. R. Leonardi, Adverse reactions caused by the use of sunscreens, Surgical & Cosmetic Dermatology, 9, 41–45 (2017). (13) V. Kostyuk, A. Pptapovich, A. R. Abuhaydar, W. Mayer, C. D. Luca, and L. Korkina, Natural substances for prevention of skin photoaging: screening systems in the development of sunscreen and rejuvenation cosmetics, Rejuvanation. Res., 21, 91–101 (2018). (14) A. Braakhuis A, Evidence on the health benefits of supplemental propolis, Nutrients, 11, 2705–2720 (2019). (15) C. Batista, A. V. F. Alves, L. A. Queiroz, B. S. Lima, R. N. P. Filho, A. A. S. Araujo, R. L. C. de Albuquerque Junior, and J. C. Cardoso, The photoprotective and anti-inflammatory activity of red propolis extract in rats, J. Photoch. Phtobio., B, 180, 198–207 (2018).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)