37 SUNSCREEN FORMULATION CONTAINING PROPOLIS MATERIALS AND METHODS MATERIALS Raw propolis samples were gifted from a local commercial beekeeper (Sabalan, Iran). Collected propolis was kept in a dry place and stored at 4°C for more studies. Galangin PLGA, polyvinyl alcohol, and cellulose membrane dialyzing tube (molecular weight cut off 12,000 g/mole) were all purchased from Sigma-Aldrich™ (St. Louis, United States). Aluminum chloride, dichloromethane, ethanol (99.7% v/v), potassium dihydrogen phosphate (KH 2 PO 4 ), dipotassium hydrogen phosphate (K 2 HPO 4 ), sodium hydroxide (NaOH), and isopropyl myristate were all provided by Merk-Millipore™ (Darmstadt, Germany). Cetomacrogol 1000® (polyethylene glycol hexadecyl ether) (Croda™ International, Snaith, United Kingdom) was provided as the gift from Croda™ International (Snaith, United Kingdom). Liquid paraffin and cetostearyl alcohol were purchased from Mojalal Co. (Tehran, Iran). Freshly prepared, deionized double distilled water was provided by using Mili-Q® ultra-pure water purification system (Merck Millipore™, Burlington, MA, United States). All other chemicals were of pharmaceutical grade and were used as received. PREPARATION OF PF EXTRACT The extraction procedure was performed according to previous studies with some modification (24,25). Briefly, 1 g of the propolis sample was added to 50 mL of ethanol and the mixture was stirred at room temperature for 72 hours. To avoid solvent evaporation, the container was covered by Parafilm® (Bernis Company, Inc., Neenah, WI, United States). To obtain a transparent solution, the ethanolic extract was then filtered through a Whatman® filter paper (Tisch Scientific, Cleves. OH, United States) using a Büchner funnel. The obtained extract was stored at 4°C for further experiments. The standardization of the extract was performed using galangin as the reference for quantification of total flavones and flavonoids (26). The spectrophotometric assay was performed based on the formation of a complex between aluminum (i.e., Al3+) and carbonyl- or hydroxyl- functional groups of the total flavones and flavanols (1). Briefly, a standard solution of galangin (100 µg/mL) was prepared by dissolving the precisely weighted amounts of galangin in ethanol. Then, a series of dilution were prepared from the stock solution to achieve concentrations of 50 μg/mL, 20 μg/mL, 10 μg/mL, 5 μg/mL, and 1 μg/mL, separately. For colorimetric assay, a mixture of test solution (2 mL), ethanol (20 mL), and ethanolic solution of aluminum chloride (5%, 1 mL) were all mixed, kept for 30 min, and the absorption was measured at 425 nm by a UV–Vis double beam equipment (Analytik Jena, SPECORD 210 PLUS, Jena, Germany). The experiment was performed in triplicate in three consequent days. The output data showed proper linearity (R2 = 0.9976) and proper inter- and intra-day precision and accuracy in the range of 1 μg/mL to 50 μg/mL. FABRICATION OF NANOPARTICLES In this study, nanoparticles were prepared by O/W emulsification and solvent evaporation method (27). Some preliminary studies were performed for optimization of nanoparticles (data not shown). All experiments were done on triplicate.
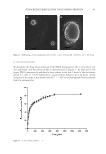
38 JOURNAL OF COSMETIC SCIENCE The optimized nanoparticles were prepared as follows: Firstly, 50 mg of PLGA was dissolved in dichloromethane (1.5 mL). Then 1 mL of the ethanolic extract of propolis (equivalent to 5.5 mg/mL of galangin) was added to the clear solution. The organic phase was heated on a water bath to 50°C and stirred on a stirrer (IKA®-Werke GmbH, Staufen, Germany) at 500 rpm. The aqueous phase was prepared by dissolving polyvinyl alcohol (2%) and was heated on a water bath to reach the equal temperature of the previously prepared organic phase. Then, the organic phase was added dropwise into the aqueous phase under a homogenizer mixer (at 15,000 rpm), and the residual organic solvent was evaporated using a Heidolph® rotary evaporator (Heidolph Instrument GmbH, Schwabach, Germany). For precipitation of nanoparticles, the prepared opalescent colloidal sample was centrifuged at 14,000 rpm for 30 minutes at 4°C. Then, the settled-down nanoparticles were collected for further experiments while the transparent aqueous phase was collected for determination of EE% and loading efficiency percentage (LE%). For better comparison, the plain PLGA nanoparticles, which did not contain PFs, were fabricated and characterized by the same procedures. CHARACTERIZATION OF THE PHYSICOCHEMICAL PROPERTIES OF NANOPARTICLES Determination of size, PdI, and zeta potential. For in vitro characterization, the settled-down nanoparticles were dispersed in deionized water and their zeta potential, particle size, and PdI were measured by dynamic light scattering technique using a nano-sizer (Nano ZS90 Malvern®, Malvern Instruments, Malvern, Worcestershire, United Kingdom) at the wavelength of 415 nm at 25°C with detection angle of 90°. Determination of EE% and LE%. The indirect method was used for determination of EE% and LE% of the nanoparticles. Briefly, the transparent aqueous phase, which was obtained following centrifugation of the freshly prepared colloidal suspension, was analyzed for determination of the amounts of free PFs that were not incorporated in structure of the nanoparticles. The previously mentioned method of aluminum chloride was used for quantification of PFs (based on the amount of galangin) in the aqueous phase. Then the EE% and LE% of the PFs, encapsulated into PLGA nanoparticles, were calculated using following equations [Equation 1, Equation 2]: EE% of initiqal PF Mass of free PF in aqeous solution) = - (Mass ×100 Mass of initial PFs (1) LE% of initial PF Mass of free PF in aqeous solution) = - (Mass ×100 wheight of nanoparticles (2) FREEZE-DRYING OF THE NANOPARTICLES For lyophilization, the nanoparticles were reconstituted using mannitol (1% w/v, 1 mL) as the cryoprotectant and were freeze-dried by an Operon® freeze dryer (Operon, Gyeonggi-do, South Korea) for a period of 48 hours. For determination of the effects of lyophilization process on physicochemical properties of the nanoparticles, the freeze-dried samples were
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)