39 SUNSCREEN FORMULATION CONTAINING PROPOLIS then resuspended in double distilled water and their size, PdI, and zeta potential were determined. The data were statistically compared with the corresponding data achieved from nonfreeze-dried samples. The experiments were performed on triplicate. MORPHOLOGICAL STUDIES For investigation of the morphology, the lyophilized samples were examined by scanning electron microscopy (SEM). The nanoparticles were set up by aluminum stubs, coated by a gold layer, and were examined using an SEM (JEOL-JSM-6360JAPAN). The SEM images were acquired in two magnifications of ×4,000 and ×20,000, separately. IN VITRO RELEASE STUDY In this study, diffusion through a dialysis bag was used to investigate the release profile of PFs from the PLGA nanoparticles (20). Briefly, the proper amounts of the lyophilized nanoparticles, containing PFs equivalent to 4.96 mg of galangin, was resuspended in a phosphate buffer (pH = 5.0). Then after, the colloidal mixture was inserted into a dialysis bag (Molecular cut off 12,000 Da). The bag was then immersed in 150 mL of a phosphate buffer (pH = 5.0) mimicking the skin condition. For increasing the solubility of PFs, TWEEN 80® (Croda Americas, Inc., Princeton, NJ, United States 2% w/v) was added to the release medium. The volume of the medium was selected in a manner that establishment of the sink condition was ascertained. The medium was shaken at 150 rpm while the temperature was kept constant at 32 ± 1°C (mimicking the skin temperature) using a Memmert® shaker incubator (Memmert GmbH, Schwabach, Germany). At different time intervals, 1.5 mL of the medium was collected and was immediately replaced by the equal volume of previously heated fresh buffer. The amounts of galangin in the collected samples were determined spectrophotometrically, and finally the cumulative release profile of galangin, as the representative of PFs, was calculated. FORMULATION OF THE SUNSCREEN For evaluation of efficacy of the nanoparticles as a sunscreen agent, the nanoparticles were incorporated into a sunscreen formulation. For this purpose, a pharmaceutical O/W placebo cream base was formulated. Briefly, suitable amounts of cetosrearyl alcohol (6% w/w) were melted in a stirrer, equipped with a hot plate. The molten was mixed with proper amounts of liquid paraffin (5% w/w) and isopropyl myristate (5% w/w). Then, the prepared oil phase was heated to the temperature of 70°C. For preparation of the aqueous phase, proper amounts of Cetomacrogol 1000® (Croda™ International, Snaith, United Kingdom 1.5 w/w) as the emulsifier was dissolved in appropriate volume of deionized water (82.5 w/w) and was heated to the equal temperature of the oil phase. The oil phase was then added gradually to the aqueous phase under stirring and the prepared O/W emulsion was kept nonagitated for a period of 24 hours to form a suitable pharmaceutical cream base. To avoid any bubble formation, the mixing process was performed in vacuum chamber. The prepared cream was evaluated visually for proper physicochemical properties such as viscosity, thickness, homogeneity, and spreadability. Then, suitable amounts of either the
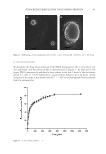
40 JOURNAL OF COSMETIC SCIENCE PFs-containing nanoparticles, free ethanolic extract of PFs, or plain nanoparticles were incorporated in the prepared cream using levigation technique. DETERMINATION OF SPF The in vitro determination of SPF of the prepared sunscreens was performed according to previous reports, with minor modifications (4). Experimentally, 1 g of the sunscreen formulation containing either free PFs, propolis-containing PLGA nanoparticles, or plain nanoparticles were weighed and were added to 100 mL of ethanol and sonicated for 5 minutes using an ultrasound bath followed by filtration using Whatman® filter papers (Tisch Scientific, Cleves. OH, United States). Next, the transparent filtrate (10 mL) was collected and reached to the volume of 50 mL by ethanol. Finally, the UV absorbances of the samples were measured separately in the wavelength range of 290–320 nm in each 5 nm intervals. The SPF was calculated according to Equation 3. SPF CF I( Abs( = ( ) ) ∑EE * * * 290 320 λ λ) λ (3) Where: • EE: Erythemal effect spectrum. • I (λ): Solar intensity spectrum. • Abs (λ): Absorbance of sunscreen extract. • CF: Correction factor that was considered as 10. In this equation, the values of EE and I in each wavelength were determined and reported previously (15). STATISTICAL ANALYSIS In this study, all experiments were done in triplicate and the results were reported by mean ± SD. Two sample independent t-tests were applied for comparison of two groups of data, whereas, for statistical analysis of more than two groups, one-way analysis of variance accompanied with Tukey post hoc was applied. The statistical analysis was performed using SPSS® software (IBM, Armonk, NY, United States, V.16.0). The significancy level was considered 0.05 in all cases. RESULTS CHARACTERIZATION OF PLGA NANOPARTICLES Zeta potential, particle size, and PdI of the nanoparticles (either propolis-containing nanoparticles or plain PLGA nanoparticles) were determined before and after lyophilization. The data are summarized in Table I. The size distribution graph of the propolis-containing nanoparticles is shown on Figure 1. As it is obvious from the table, the particles exhibited proper physicochemical properties.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)