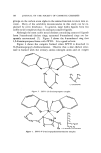
104 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS groups on the carbon atom alpha to the amino function is taken into ac- count. Many of the solubility inconsistencies in this study can be ex- plained by steric hindrance. In general, large bulky ligands form less stable metal complexes than do analogous smaller ligands. Although the most stable metal chelates containing saturated ligands form 5-membered chelate rings, saturated 6-membered rings are fre- quently encountered (7). Figure 3 shows the 6-rnembered ring that 1,3-diaminopropane forms with ZPTO. It is a good solvent. Figure 4 shows the complex formed when ZPTO is dissolved in N-(3-aminopropyl)-diethanolamine. Observe that a zinc chelate struc- ture is formed with the tertiary amino nitrogen atom and an oxygen Figure 3. ZPTO-1,3-diaminopropane complex C H•C H• 0 H
ZINC PYRITHIONE PREPARATIONS 105 atom of one of the hydroxyl groups. It was concluded that the pri- mary amino nitrogen atom does not participate in forming the chelate structure. The experimental proof for this is that when the analogous compound without hydroxyl groups, N,N-diethyl-l,3-propanediamine, was tested for its solvent capacity on ZPTO, the solubility of ZPTO was reduced from well over 10% at 20øG to less than 1%. Diglycolamine The superior solvent power of diglycolamine could be ascribed to the formation of a tetradentate chelate structure with ZPTO (Fig. 5). •k• •.,• . . • ...... • DIGLY Figure 5. ZPTO-diglycolamine complex Diglycolamine, which may be thought of as a derivative of diethyl ether, was chosen as the solubilizing agent for ZPTO in several clear formula- tions (Table I). These formulations are limited by the low water concentrations that are required for clarity. Increasing the concentra- tion of the water causes the disruption of the ZPTO-diglycolamine com- plex permitting free ZPTO to be thrown out of solution. The proto- type hair-setting lotion formulation that was prepared (Table I) con- tains 0.2% ZPTO and is clear. When water is added it becomes cloudy due to liberated ZPTO. The pH of a 1:1 mixture with water is 8.8. The shampoo preparation shown in Table I has good foaming proper- ties due partly to the high diethanolamide content. As expected, the
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)