300 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Foams vary in their physical behavior. Wet foams, meaning foams of high liquid content, are likely to drain rapidly, unless the liquid vis- cosity is high. Similarly, wet foams are likely to be internally mobile. The high liquid content minimizes interbubble contact and packing, thus allowing movement (slippage) between bubbles. In the extreme case, when the liquid content is so high that the bubbles are completely spherical and mobile except for liquid viscosity effects, the dispersion is no longer a true foam but is rather a "gas emulsion" (1). If sufficiently stable, a wet foam will drain to become a dry foam, meaning a foam of low liquid content. In a dry foam, the bubbles press together to form blunted polyhedra. Interbubble slippage is then mini- mal. However, an increase in the degree of inhomogeneity in bubble size can shift the behavior of dry foam toward that of wet foam, especially in the matter of mobility. Small bubbles can fit among larger bubbles, thus diminishing the flattening of the bubble walls. This, in turn, re- duces the tendency to form polyhedra and, consequently, loosens the pack- ing and increases the mobility of the bubbles. Based on geometric considerations for close-packed spheres, bubbles of uniform size should be mobile when the liquid content of the foam exceeds 26% by volume. However, in practice, bubble mobility can oc- cur at substantially lower liquid fractions, chiefly because of actual in- homogeneity in bubble size. In a vertical glass column of rising foam produced by bubbling gas through a spinnerette submerged in a liquid pool at the bottom, bubble mobility in the foam was reported to begin at a superficial gas velocity of about 160 cm/min, corresponding to breaks in the plots of pressure drop and foam liquid carryover versus gas rate (2). When bubble mobility is absent, the foam ascends in plug flow by slipping readily along the glass walls (3). The bubbles themselves can be produced by sparging (bubbling gas through) a liquid pool, by the release of dissolved gas, by the chemical production of gas, or by mechanical means such as shaking or beating. The resulting bubble sizes depend on complex hydrodynamic factors. For foams generated by a high-speed mixer, the distribution of initial bubble sizes may be approximated by eq 1, 6 br• F(r,) = (1 q- br,2) 4 (1) where F(r•) is the frequency distribution function, re is the bubble radius, and b is the distribution parameter (4). Bubbling prehumidified nitro-
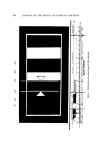
PHYSICAL ASPECTS OF FOAM 301 gen through an extra-coarse sintered-glass sparger (0.02-cm openings) into a 12-1. flask of bovine serum albumin in water at a concentration 3.6 times the critical micelle concentration resulted typically in a foam with bubbles of approximately 0.1-cm average diameter with a standard devia- tion of 0.05 cm (3). The polyhedral bubbles of a dry foam fit together in such a way that their common walls meet three at a time, theoretically at angles of 120 ø. This is the stable condition for balanced forces. The junction of the three walls constitutes a channel or capillary often termed a Plateau bor- der (abbreviated PB), as shown in Fig. 1. The PB's intersect four at a t•me to form tetrahedral angles of approximately 109 ø in space. ß Figure 1. Three bubble walls (fihns) intersecting to form a Plateau border (PB) The curvature of the PB walls produces a suction in the PB according to the equation of Laplace and Young, /1 1\ 6p =q, •} q-/•2) (2)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)