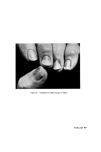
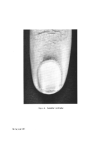
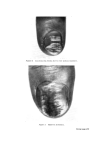
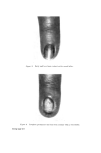
STRUCTURAL ASPECTS OF KERATIN FIBRES 433 protein is also very different from that of whole wool in that it contains smaller amounts of cystine, proline, threonine and serine and larger amounts of lysine, histidine and the aromatic amino acids (26). The cortex Proceeding inwards from the cuticle, the major structural feature of the fibre is reached, namely, the cortex. Horio and Kondo (34) noted that the cortex of fine wool fibres has a bilateral structure, in the sense that about one-half of the cortex always absorbs a given type of dye more intensely than the other half. Numerous reactions indicate that the more reactive side always lies on the convex side of the crimp wave. The side with greater reactivity is called the orthocortex and that with lesser the paracortex. This asymmetry between the orthocortex and paracortex has been shown in differences in morphological appearance (30, 35), staining behaviour (36) and certain chemical and physical properties (37). In bilateral wool fibres, the sulphur content of the paracortex is appreciably higher than that of the orthocortex (38). There are more cystine, proline, and glutamic acids but less glycine, phenylalanine, and tyro sine in the former than in the latter (28). The presence of a higher content of the cross-linking cystine would account for the lower degree of swelling of the paracortex, its greater resistance to acid hydrolysis and the slower rate of reduction of its cystine (39). Within each cortical cell of wool, which is about 80 •mlong and 5 •m in diameter, there is great complexity of structure. Electron micrographs of stained transverse sections of wool show that the cortex consists of approxi- mately circular macro fibrils (also referred to as 'tertiary aggregates' of the a-helices) having the appearance of whorls or spirals. Within the macrofibril are microfibrils (also referred to as 'secondary aggregates' of the a-helices) about 7.5 nm in diameter arranged in pseudo-hexagonal packing and embedded in a more heavily stained amorphous matrix.* The microfibrils themselves consist of a number of protofibrils (also referred to as 'primary aggregates' of the a-helices) about 2 nm in diameter arranged in a regular manner and packed within the micro fibril and embedded in intramicro- fibrillar matrix protein (40, 41). Independent support for the concept of *The electron-opaque appearance of structural units after fixation with metal compounds has been interpreted as an intense reaction of--SH or --S--S-- bonds with the metal, whereas an electron-translucent appearance is considered to be a weak reaction. Accordingly, the dense region seen in the cortex is thought to indicate the presence of amorphous protein(s) stabilized by numerous --S--S-- bonds, while the less dense region reveals the presence of a fibrous protein stabilized by less numerous --S--S-- bonds.
434 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS a protofibrillar substructure has been obtained by several investigators (42) who succeeded in isolating fine filaments, about 2 nm in diameter, from e-keratin preparations fragmented by ultrasonic irradiation. However, it is contended that the filaments observed in these preparations almost cer- tainly result from cellulosic contaminants (41, 43). Moreover, experiments by Sikorski and associates (44) on the interpretation of high magnification electron micrographs of keratin fibre sections indicate that the distinctions between the microfibrillar patterns of various cortical cells may not be as simple as has been indicated. They regard the 'microfibril' as a manifestation of an average mode of aggregation in situ of protofibrils. The medulla The medulla is a histological component situated near the centre of the fibre in many keratin fibres such as human hair, but it does not occur in fine wool fibres (45). It is formed from an axial stream of cells, the contents of which shrivel up during dehydration leaving a series of vacuoles along the fibre axis. Many variations occur in the shape and size of this part of the fibre. It has been usual, however, to concentrate studies on wool fibres in which medulla is either absent or present in only small amounts. In any case, the medulla is believed to make little contribution to the chemical and mechanical properties of the fibre. That the material of the medullary cells differs from that of the surrounding cortical cells is indicated by the differences in staining characteristics of the two types of cells. Recently, amino acid analyses of the separated medullary cells from rabbit, kangaroo, and platypus hair show that they contain about 1 residue in 4-5 of glutamic acid, 1 in 9 of citrulline, 1 in 12 of leucine, 1 in 14 of glycine and only 1 in 35 of cystine (46). The citrulline is covalently bound in the peptide linkage in the proteins (47). It has been shown that the presence of N6-q, - glutamyllysine cross-link in hair and quill medulla protein of mammalian species is a general phenomenon (48). The structure of human hair An insight into the histological structure of human hair has been gained from the electron microscopic observations in this laboratory of sections of ether-degreased fibres stained with various heavy metal compounds (49). Human hair, like most other keratin fibres, consists of a central core of long spindle-shaped interdigitating keratin-filled cortical cells this core is bounded by a sheath of overlapping leaf-like cuticle cells. Perhaps the most
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)