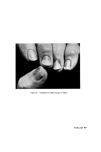
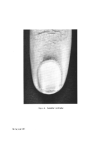
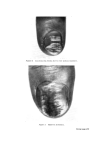
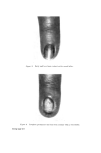
438 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ISOLATION AND CHARACTERIZATION OF PROTEIN FRACTIONS FROM KERATIN FIBRES In the last 15 years, numerous attempts have been made to fractionate wool keratin into homogeneous protein fractions without main chain hydrolysis. In general, two main kinds of protein fractions could be obtained, i.e. the so-called low-sulphur and high-sulphur protein fractions. Oxidation of the disulphide bonds of keratins with peracetic acid (77) or performic acid (78) and extraction with ammonia solution leaves an insoluble residue termed [•-keratose. The low-sulphur fraction, a-keratose, is precipitated by acidification of the filtered extract and the high-sulphur fraction, T-keratose, remains in solution. By keratose estimations on wool, human hair, and some isolated cell components, Asquith and Parkinson (79) have provided chemical confirmation that a-, [•-, and T-keratoses are to be identified respectively with fibrillar, cell membrane, and matrix components of the fibre. Similarly, reduced and subsequently carboxy- methylated wool gives the S-carboxymethylkerateines (SCMK), which can be fractionated to give SCMKA low-sulphur fraction and SCMKB high- sulphur fraction (80). a-Keratose and SCMKA are heterogeneous low- sulphur protein fractions of almost identical amino acid composition (81), while T-keratose and SCMKB are extremely heterogeneous high-sulphur protein fractions with similar but not identical am/no acid composition (82). The low-sulphur fractions are relatively rich in aspartic and glutamic acids, lysine, tyrosine, leucine, and alanine, while the high-sulphur fractions are poor in these amino acids but contain relatively large amounts of cysteic acid or S-carboxymethylcysteine, proline, serine, and threonine. Studies on these protein fractions are yielding useful information about the molecular structure on keratin fibres. In [•-keratose, a substantial amount of the lysyl amino group has been found to exist naturally as Nø-T-glutamyllysine cross-link (83) and in T-keratose, 83•o of the glutamic or aspartic acids are present as their amides (84). Seasonal factors have a marked influence on the proportion of keratoses in wool keratin during maintenance of sheep on pasture, the percentage of T-keratose increases while in the wool grown during the winter stall-maintenance, a-keratose increases (85). In the SCMKA fraction the carboxyl groups are present mainly as glutamic acid rather than aspartic acid residues, whereas in the SCMKB fraction there is a relatively high content of glutamine (11). It has been shown that low-sulphur SCMKA kerateines contain about
STRUCTURAL ASPECTS OF KERATIN FIBRES 439 50•o helix while high-sulphur SCMKB kerateines are almost completely devoid of helix (86). Partial digestion of the low-sulphur SCMKA fraction from wool gives rise to helix-rich material (87, 88). Using Davies' graphical method (89), Asquith and Shaw (90) have calculated that low-sulphur e-keratose contains 29•o (Val + Ile + Ser + Thr + Cys) and hence is about 40•o e-helix, and that high-sulphur I•-keratose contains 53}/0 (Val + Ile + Ser + Thr + Cys) and hence is non-e-helix. Although the helical regions of the low-sulphur SCMKA fraction contain most of the lysine residues of the wool fibre, they are highly anionic because they also contain most of the free carboxyl groups. The non-helical sections in the SCMKA fraction are cationic (88). From the amino acid composition and physical measurement data, it would appear that the low-sulphur fractions are derived from the proto- fibrils and the high-sulphur fractions from the intermacrofibrillar matrix. It is generally agreed that the protofibrils consist of several e-helical poly- peptide chains twisted around one another in a rope-like manner, and it is essentially this ordered structure which gives rise to the X-ray diffraction pattern of e-keratins. On this basis, the matrix protein is considered to be disordered, at least in so far as there is insufficient long-range order to give rise to an X-ray diffraction pattern. The matrix is more heavily stained by metals than the protofibrils and this would seem to indicate that the former contains more cystine. However, it is not yet possible to separate unequivo- cally the matrix protein from the protofibrillar protein, which is not sur- prising in view of the obvious difficulties involved. Thus, all evidence with regard to the identity of the proteins of the matrix and the protofibrils is of necessity indirect (6, 91). The fractionation and separation of a single protein molecular species from such a mixture has been the subject of extensive research. The work of Lindley, Gillespie and Haylett (92) on a protein from SCMKB-2 high- sulphur protein fraction shows the occurrence of a high frequency of homodipeptides such as Pro-Pro, Val-Val, Glu-Glu, and Cys(CH•CO•H)- Cys(CH•CO2H). By partial acid hydrolysis of [asS]-cystine labelled wool, the Cys-Cys sequence is shown to occur frequently (93). Asquith and co- workers (94) have also found that Cys(SOaH)-Cys(SOaH) is a very import- ant sequence in T-keratose, over 30•o of the cysteic acid occurring in this sequence, and thus have postulated that a large proportion of the lanthionine formed in wool under suitable conditions is intramolecular. Recently, a polypeptide containing 97 amino acid residues, of which 26 are aromatic (tyrosine and phenylalanine) and 24 are glycine, has also been
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)