586 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ß 40 20 PER CENT STEROID SOLUBILIZED IN VEHICLE Figure 9. Correlation o[ per cent corticoid solubilized in vehicle with in vitro release and in vivo vasoconstriction ß In vitro total release at 7 hours (q,/cm =) ß In vivo vasoconstriction, open application (% sites responding at 32 hours) single patch of chronic stable psoriasis, allowing for an unusual number of "controls" per experiment. Evidence of effectiveness is measured by nothing less than complete suppression of the psoriatic epidermis--an es- sentially objective "all or none" end point. Clinical Evaluation Clinical Trials The final step in the clinical testing program is a face-to-face evalua- tion of our product versus accepted standard treatment regimens and competitive products in actual dermatoses. The clinical efficacy of fluocinonide in the FAPG vehicle was established by clinical trials utiliz- ing double-blind, paired comparison studies of various dermatologicaI disorders such as psoriasis (38). In psoriasis, when compared to a commercially available hydrocorti- sone 0.5% cream, fluocinonide 0.05% FAPG Cream produced a su- perior clinical response in 10 pso?iat.ic patients out of 13 in one study and 70 out of 102 in a second study (Table II). These results are statistically significant (p 0.01).
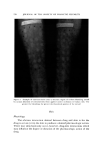
CORTICOID, VEHICLE, AND SKIN INTERACTION Table II Performance of Lidex© • (0.05% Fluocinonide in FAPG ©,• Cr3am) in Treatment of Psoriasis in 242 Subjects 587 Study A Study B Study C Tot,l Lidex Compared to W/L/T o W/L/T W/L/T W/L/T Placebo 13/1/lc 13/1 Hydrocortisone 0.5% 10/0/3c 70/12/20• 80/12/23 Betamethasone valerate 0.1% 7/5/18 47/6/29c 54/11/47 a Syntex Laboratories, Inc., Palo Alto, Calif. 0 W denotes greater improvement with Lidex, L denotes greater improvement with com- parative material, T denotes no difference. ß Statistically significantp 0.01. Figure 10 shows one of many psoriasis cases which have benefited from our efforts. A major point to remember is that topical corticoids, such as hydrocortisone, had been unable to significantly influence this disease until the advent of their fiuorinated derivatives. These compounds were particularly effective in psoriasis when used under occlusive dressings. With the development of fiuocinonide and properly designed vehicles, the same dramatic results can now often be achieved without occlusive dressings. Analysis Sequential analysis has recently been utilized to scrutinize results as they become available and allows termination of the trial as soon as sta- tistical significance is reached (39). In fact, however, we frequently con- tinue gathering case reports so that we can better evaluate drug safety. CONCLUSION A long and increasingly difficult path must be followed in the devel- opment of new, effective medications. We have outlined just some of the steps which are followed in our research and development labora- tories during the creation of new topical corticoids (4'0). It is certainly a far cry from the early days where a new chemical moiety was pulverized and incorporated into a nice, white cream which was the favorite of the production supervisor. We have not even touched upon the many ana- lytical problems inherent in establishing the chemical integrity of minute concentrations of these increasingly sophisticated molecules in unusual and complex vehicles, as well as in the media of our release and penetra- tion studies. Nor have we touched upon vehicle aesthetics, packaging stability, and preservation problems which would have been our prime
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)