SKIN IRRITATION BY ANIONIC SURFACTANTS 51 4.0 12 12 C12 MAP mono Na TEA 36 12 6 6 6 6 12 12 6 6 6 12 6 12 number tested C!2 LAS C8 C12 C14 C16 AOS ES C12 C8 CI0 C12 C14 C12 AS L A S 2 S O A P 14 ES EO Figure 8. Skin-roughening potency of monoalkyl phosphates compared to various common surfactants shown by average skin roughness score which was calculated shown by dividing the sum of the numerical values given to the severity of roughness and to the number of treatments necessary to produce the onset of roughness, by the total number of subjects tested. MAP: monoalkyl phosphate, AS: alkyl sulfate, LAS linear alkyl benzene sulfonate, SAS: paraffin sulfonate, AOS: alfaolefin sulfonate, ES: laury ether sulfate, Soap: sodium carboxylate, EO: lauryl polyoxythlene. severe roughening, followed by C14LAS, AOS, C10Soap and C•.4Soap, whereas C•2-14EO and CsSoap produce the least roughness. Of all surfactants tested, the MAP belongs to the last group, suggesting that the roughness-inducing effects are similar to those of general nonionic surfactants such as C12-14EO. SOME POSSIBLE MECHANISMS FOR LOW ROUGHENING OF MAP Many factors, such as removal of skin surface lipids (5), loss of naturally occurring hygroscopic materials in horny layers (6), adsorption (4,7), protein denaturation (8,9), epidermal lysosomal injury (10), and repeated irritations, have been reported to have an influence on the pathogenesis of skin roughness. Common dishwashing procedure generally consists of not only limited duration of contact between skin and surfactant but also subsequent rinsing off of the surfactants with running water. It is possible, therefore that the above described pathogenic factors can be classified into at least two groups of action, viz. 1) occurring soon after washing such as removal of skin surface lipids, loss of hygroscopic materials, and adsorption, and 2) protein denaturation and membrane injury which may occur at later period. Of the primary pathogenic factors described above, we have found in an in vitro study (7) using callus powder that adsorbing residual effect of surfactants was particularly comparable to the induction of the skin roughness (2).
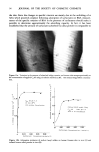
52 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS EoQ D -65 ///• ô ......... • a C• AS i C. •AOS •/-'•m • C•nAS j ClazAOS c C' •AS k C ' =AOS 12 16 d C14AS m C18AOS e C. LAS zf c•s ñu c I2•s 12 hg C.. LAS C16LAS ...................... 0.5 l•0 1.5 ( l•3mole/g ) Adsorption by Callus ( 1,0%, 40C, 2 hr ) Figure 9. Relationship of adsorbing ability onto skin measured by adsorbed amounts of surfactants onto isolated callus after incubation of 1% concentration for 2 hr at 40øC., and specific rotation of BSA in the presence of the 1.5% surfactant. Note that there is a positive correlation (r=0.93) with a significant difference of 95%. AS: alkyl sulfate, LAS: linear alkyl benzene sulfonate, AOS: alfo-olefin sulfonate. -651 - 60 -55 o CI0 AS ß C•18F D t10 MAP monona nC8 %_,/. 0 5 1.0 1.5 Snrfactant Concentration( g /100ml) Figure 10. Changes in specific rotation of BSA plotted against the concentrations of monoalkyl phosphates. AS: alkyl sulfate, MAP: monoalkyl phosphate.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)