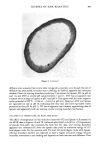
AMINE ADSORPTION ON KERATIN 291 Adsorption of Lonc]-choin Monoomines •fion / oeou[ombic offroof ion • l•1t•1111i•111,t• - Fiber Surfoce I I !•111•111GI Mono[oyer I•1111•11 lel l l f•l Hemi-miceile Hydrophobic offroof ion Adsorpfion of Long-choin o(.,(u-Diomines Figure 3. Schematic representation of possible modes of adsorption of long-chain monoamines and c•, o0-diamines at a keratin fiber surface. charged groups as opposed to the vertical arrangement in the case of the monoamine, then subsequent aggregate formation by way of hydrophobic interactions (discussed later) would result in a comparatively lower uptake per unit surface area. ANALYSIS OF ADSORPTION ISOTHERMS AT CONSTANT pH Figure 4 shows the adsorption isotherms (at 40 ø) on wool of dodecylamine and 1,12-diaminododecane at pH 8 where each point represents the equilibrium distribution of sotbate between the fiber (Cf, mM/Kg dry fiber) and the solution in contact with the fiber (Cs mole/liter). Although it is more usual to represent concentration as a linear function, the isotherms shown in this study display concentrations of adsorbate as a negative logarithm. This mode of presentation was adopted in order to detect small differences in adsorptive behavior at low concentrations. When the isotherms were plotted with Cf as a function of Cs (mole/l), the shape of the curve could be classified according to the phenomenological scheme of Giles (21) as Class L, Subgroup 2. To test whether the isotherms shown in Figures 4 and 5 conform to ideal behavior in which monolayer formation results from adsorption at specific sites of equal energy, an attempt was made to fit the isotherm to the Langmuir equation. Two graphical plots were made: 1/Cf against 1/C s (Klotz plot) and Cf/Cs against Cf [known as the Scatchard plot, the form most frequently used in theoretical analyses of binding interactions in biopolymer systems (22)]. In neither case was linearity observed, which leads to the conclusion that the conditions mentioned above regarding monolayer adsorption are
292 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 200 v 150 E ø• • o .o •-"' ø••H2N- [ CH2} •'•N H 2 oiO ß 0 I I I I I I 4.5 4.0 3.5 3'0 2.5 2'0 -log [equilibrium concentration} Figure 4. Adsorption isotherms (40 ø) of dodecylamine (Ct2-NH2) and diaminododecane (H2N-(CH2)•2- NH2) on wool at pH 7.8. 400 0 •0 -- 350 /o o • 300 • o "" 250 •. C12H25NH2 E -- 200 (I o 150 -r- 100 o • 50 0 ' • • • : • , • t , t t , , , , , , , , , 4'0 3-5 3'0 2'5 2-0 -log {equilibrium concentration} Figure 5. Adsorption •sotherms (40 ø) of dodecy]amine (C•2-NH•) and diaminododecane (H2N-(CH2)•2- NH2) on wool in 0.1 • NaC1 at pH 7.8.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)