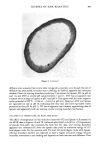
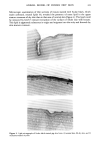
KINETICS OF HAIR REDUCTION 315 It seems possible that the tendency of DTT and lipoate to form moving boundaries at all reaction conditions can be explained by the ability of these compounds to form a dithiolane (5-membered) or dithiane (6-membered) ring structure on oxidation. The ring structure stabilizes the oxidized molecule even though these rings are strained (2,4,6). This stabilization shifts the reduction equilibrium, and the presence of any reducing agent in the hair causes considerable reaction, greatly increasing the permeability of the hair, leading to the formation of the moving boundary. TG is a small molecule and is not an efficient reducing agent much below pH 10. Thus it can diffuse through the hair to a considerable extent before any significant reaction takes place, leading to pseudo first-order kinetics. Sodium bisulfite behaves in a similar fashion (1). At pH 10, TG is a very efficient reducing agent and also forms a moving boundary. The work reported here is of a basic nature, but the SFTK method could be used for more development-oriented studies such as comparing the efficacy of hair waving solutions. By using different sections of the same hair, the efficacy of reducing solutions could be compared by simply determining the per cent of reduction in tensile force at a given time. This method would not require extensive data analysis and does not rely on the assumptions that went into deriving equations 3 and 5. The SFTK method has two important advantages over the recently reported "hair loop test" (7) which is similar in principle. Our method uses much less hair than the hair loop test so different sections of the same hair can be used for comparisons, and information about the reaction mechanism can be inferred from the shape of the SFTK curves. The SFTK method should be applicable to studies of disulfide bond reduction in wool, and could be used to probe changes in the permeability of keratin fibers that may have occurred as a result of other treatments by determining the effect on reduction rates. ACKNOWLEDGEMENTS The strain cycle procedure was suggested and developed by Mr. James Innis. The micrographs shown in Figure 5 were obtained from Dr. Bruce Barman and were taken in the Microscopy Department of the Miami Valley Laboratories by Ms. Janet Van. REFERENCES (1) C. E. Reese and H. Eyring, Mechanical properties and structure of hair, Textile Res. J., 20, 743-750 (1950). (2) W. W. Cleland, Dithiothreitol: A new protective reagent of SH groups, Biochemistry, 3, 480-482 (1960). (3) J. Crank, The Mathematics of Diffusion (Oxford University Press, 2nd Ed., 1975), pp 298-313. (4) H. D. Weigman, Reduction of disulfide bonds in keratin with 1,4 dithiothreitol 1. Kinetic investigations,J. Polymer Sci., A 1:6, 2237-2253 (1968). (5) L.J. Wolfram, Reactivity of disulphide bonds in strained keratin, Nature, 206, 304-305 (1965). (6) U. Schmidt, P. Grafen, and H. W. Goedde, Chemistry and biochemistry of c•-lipoic acid, Angew. Chem. Internat., Edit/Vol. 4, 846-856 (1965). (7) J. Szadurski and G. Erieman, "The hair loop test," Cosmetic Science and Technology, Vol. II (IFSCC 12th International Congress, Paris, 13-17 September 1982), pp 391-405. (8) R. R. Wickett, unpublished results.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)