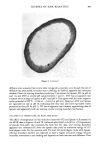
KINETICS OF HAIR REDUCTION 303 1.0 0.8 I I I I I I Time (minuoees) Figure 1. SFTK curves for thioglycolate (TG) and 1-4 dithiothreitol, 0.3 M thiol, pH 9.0, 25øC. tensile stress loss is much faster with DTT than with TG, and the curves have very different shapes. The DTT curve starts out slowly and then accelerates before tailing off, while the TG curve is typical of single exponential decay. In order to derive mathematical models for the kinetic processes, we assume that each labile, stress supporting, disulfide bond supports the same percentage of the tensile stress and has the same intrinsic rate of cleavage by a given reducing agent. The additional assumptions required depend upon the nature of the proposed model. Two models have been derived: a pseudo first-order model for reaction conditions producing SFTK curves similar to the TG curve in Figure 1, and a moving boundary model to describe curves similar to the DTT curve. To obtain the pseudo first-order model we assume that the diffusion of the reducing agent through the hair is fast compared to the rate of reduction and that the reducing agent is present in large excess. The rate of bond breaking is: 1. d(S-S)/dt = kCo(S-S), where Co is the concentration of reducing agent, and (S-S) is the instantaneous concentration of intact disulfide bonds. The back reaction is negligible because of the presence of a large excess of reducing agent. The tensile stress at any time, F(t), is assumed to be directly proportional to (S-S) and the solution of the SFTK rate equation is: 2. F(t)= F(0)exp(--kC0t).
304 .JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Reese and Eyring (1) originally derived equation 2 and showed that reduction of hair by sodium bisulfite follows pseudo first-order kinetics. If equation 2 correctly describes the reaction, plots of --ln(F(t)/F(0)) versus t will be linear with a slope of kC0 and an intercept of zero. Figure 2 shows such plots for SFTK data from TG at pH 9.0 and two different concentrations. The linear fits are excellent with intercepts near zero as predicted. The rate constants obtained from the analysis agree with 5%. These results provide good evidence that reduction by TG is obeying a pseudo first-order rate law under these conditions. 0.õ 0.4 ,-,0. 0.1 •ime Figure 2. Thioglycolate SFTK data fit to pseudo first-order kinetics. 1.0.16MTG, k =4.1 x 10 4M •sec • 2.0.32 M TG, k = 4.3 x 10 -• M -• sec -• To derive the moving boundary model, we assume that diffusion into unreacted hair is much slower than the reaction rate, and that the reaction greatly increases permeability of the hair toward the reducing agent. Under these conditions a moving boundary of reducing agent will be formed in the hair. On the inside of the boundary the concentration of reducing agent is assumed to be zero, and on the outside of the boundary the concentration is Co. In this case the distance, X(t), that the boundary has moved into the hair at time, t, can be approximated by: 3. X(t)= At •/2, where A is a constant (3).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)