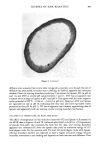
D&C RED NO. 9 ANALYSIS AND PURIFICATION 275 Reduction I Comp ønent II H3C C-•N•=-N O-• 1-(3-chloro-4-tolylazo)-2-naphthol OH NH• , 0 10 2'0 Retention time (minutes) Column TSK LS-410, 4ram •.d x 25½m, Temp.: 30øC Eluent 1MKCl, 7.5%IPA/H•), pH ----1.5 wfl:h H•PO• Detector UV (210nm) Figure 3. Identification of isolated component (d). IDENTIFICATION, SYNTHESIS, AND QUANTITATION OF SUBSIDIARY COLORS The impurities were extracted from D&C Red No. 9 by chloroform in a soxhlet extractor. The extract was roughly divided into three fractions by numbers of substituents under condition I by HPLC as shown in Figure 1. Each fraction was separated under condition II by HPLC. Then, each component was analyzed by GC-MS. They were all identified as aromatic azo compounds from their mass spectra, as shown in Figure 2. Among these components, "c," "d," and "e" give the same mass spectrum therefore, it is assumed that they are positional isomers. In order to determine the substituent positions, each isolated component was reductively cleaved by titanium trichloride to obtain two derived aromatic amines which were then identified by HPLC. For example, the liquid chromatogram of the materials to which the isolated component "d" was reduced is shown in Figure 3. It was found that "d" is 1-(3-chloro-4-tolylazo)- 2-naphthol. The structural formulae of the other isolated components were also confirmed by a similar procedure. Table I Structural Formulae of Subsidiary Colors in D&C Red No. 9 (1) P-O (2) P-6 (3) P-7 (4) P-9 (5) P-12 (6) P-13 (7) P-14 (8) P-15 ,. ,., c• Iel P4. I•0l P.17 {.)
276 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS CH 3 Red Lake C Amine diazotization CH3 diazonium salt I coupling I CH 3 O• I D&C Red No.8 SO 3Ba/ OH , /2 C N--N 8-naphtho 1 CH • • D&C Red No.9 By the analysis of D&C Red No. 9, eleven subsidiary colors shown in Table I were confirmed. Among these impurities was 1-phenylazo-2-naphthol (P-0) which is reported to be a contact sensitizer. The subsidiary colors are methyl- or chloro-derivatives of the basic skeletal structure (phenylazo-naphthol) of D&C Red No. 9. D&C Red No. 9 is the compound produced by diazotization of Red Lake C Amine and by coupling with Table II Synthesized Aromatic Azo Compounds 3 2 OH Subttltuentl and Starting materials • their position Yiek • 2 3 4 5: 6 Materials for Coupling (g) : dl•zotization compound P-0 R©age•t (Sudan ! ) / P-1 CI o-Chioroaniline •-naphthol 1.0 Po2 CI m-Chloroaniline # 1.1 P-3 Ci )- Chloro•niiine # 0.9 p-4 CH=: Reagent (O•ange SS) / P-5 iCI-b m-Toluidine •-naphl h4d 4• .. .... P-6 CI-b 3- Toiuidine # 5• P-7 CI •.H•' 2-Chioro-3-nilrotoiue•e # 0•5 P-8 CI 'CI-b' 3-Chioro-4-nilrotoiu• # 5.4 P-9 CI. .CH3 $-Chiozo-m-loiuidine # 2.4 P-10 Ci Cl• $-Chiozo-o-toiuidine # 3• Poll •-b i CI 3-Chioro-o-toluidine # 4.0 P-12 ! CI :.CH=i 3-Chloro-p-loluidlne 3•) Po13 'CI: iCI.• 4-Chlo•o-2-nitzoloiue•e # 4-• , .. P-14 .•1•. ! CI 4oChlo•o-o-toluidirte # •.0 P-15 .C•bi CI . 4-Chlotoom-loiuidlrte # 3.9 P-l• CI ' :CI •i Z4-1:Nchloro-5-nltrotoluene ,, 0.4 l•-17 ß CI iCI-b: i CI 2.S.-I:Nchlol•.M-nltrotoluene # 2• F)-18 . CI i CI '• 3.4-D•chlMo-&-nltrotoluene ,, 1:) , , , i
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)