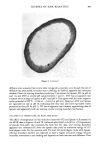
AMINE ADSORPTION ON KERATIN 295 EFFECT OF ADDED ELECTROLYTE ON THE ADSORPTION BEHAVIOUR It is believed that the anions which comprise the lyotropic series show a progressively greater ability to disrupt the hydrogen-bond network of liquid water. Thus for the series of ions--thiocyanate, nitrate, chloride, acetate, and sulfate--the thiocyanate ion has the greatest structure-breaking ability, while sulfate is thought to enhance the cohesion of water hydrogen bonds. The intermediate ions of the above series show a progression in their water-structure-breaking efficiency. Several adsorption studies of dyes on fibers, e.g., Direct Blue 1 on viscose rayon (25) have shown that the adsorption uptake at equilibrium can be strongly influenced by the presence of ions that disrupt water structure around hydrophobic sections of the dye molecule. The series of isotherms shown in Figures 6 and 7 display the extent to which the ions of the lyotropic series influence the uptake of dodecylamine and diaminododecane on wool. 600 500 400 300 200 100 ....... THIOCYANATE /. ./?•..•-.-•-•2"-•-' ..... A NITRATE I I [ i I I 0 2.5 2-0 / / [] / ! I / I I //..///e .// /// ,,ff'•// / // ?' •,,,/ ........... I I I I I I I I I I I I I I I I I I I I /.'5 4'0 3'5 3'0 -log [•quiiibrium ½on½•nJroJionl Figure 6. Adsorption isotherms (40 ø) of dodecylamine on wool at pH 7.8 in the presence of various anions. The adsorption isotherms for the monoamine (Figure 6) in the presence of various anions demonstrate the effectiveness of the water-structure-breaking ions such as thiocyanate and nitrate relative to chloride [which is believed to have little influence on water structure (26)] in promoting the adsorptive capacity of the fiber, while the action of acetate appears to be somewhat anomalous in that a maximum is apparent in the uptake. The adsorption isotherms for the diamine in the presence of the same anions (with the addition of sulfate which could not be tested with the monoamine because of amine precipitation) are shown in Figure 7. In this series it can be seen that it is the chloride ion which causes the maximum saturation of uptake. If, however, the adsorption
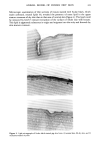
296 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 120 • 100 • 8o E • 6o õ 4o '•. .o ._ ._ • 2o r, o CHLO o /// / // o/// / ,' •- * ß SULFATE /// /•_....•.'"'"'"•/./ /' .....½_ • -_..•.__.•• •..---. t- ,••-'•-'•-'"- -'-T'-, , , , , , , , , , , , , , , 4'0 3• 3'0 2-5 2'0 -log Jequilibriurn concenJraJion) THIOCYANATE NITRATE IA) ACETATE I b) Figure 7. Adsorption isotherms (40 ø) of 1,12-diaminododecane on wool at pH 7.8 in the presence of various anions. promoting tendency is examined at a lower concentration, e.g., -log C -- -3.2, then the same order of effectiveness is observed as found with the monoamine viz. CNS- NOD- C1-. The influence of SOft is according to expectation from its position in the lyotropic series, whereas the effect of acetate is anomalous in that the isotherms in the presence of acetate and nitrate are virtually identical. The way in which anions of this series promote adsorption of alkylammonium ions may be based in the action of enforced ion-pairing of the charged head group with the less hydrated, larger anions such as thiocyanate and nitrate. These ions would be expected to adsorb preferentially to the fiber, thereby imparting negative charge to the surface the surface charge in turn attracts the cationic ammonium ion. Explanations of this nature have been used to explain the enhanced binding of dodecylammonium ions by thiocyanate to nonionic water-soluble polymers such as polyvinylpyrrolidone (26), and also the lowered affinity of Orange II for silk (27). In the latter example, the adsorption of CNS- to the fiber surface provides a charge repulsion with respect to the dye sulfonate group. A further explanation which would account for the increase in adsorption uptake affected by thiocyanate and nitrate, may be the ability of these ions to distrupt the structure of adsorbed water molecules at the solid/liquid interface. In such an event the adsorbate molecules would be able more easily to displace the layer of adsorbed water from the liquid-solid interface. EFFECT OF TEMPERATURE ON SATURATION ADSORPTION UPTAKE The isotherms for the C12 monoamine and diamine were determined at pH 8 in the presence of 0.1 M NaC1 at 40 ø, 60 ø, and 80 ø. The family of curves for these two adsorbates is shown in Figures 8 and 9 respectively. It can be seen that the equilibrium uptake of the amine at saturation is constant over the studied temperature range, from which it can be concluded that the enthalpy of adsorption is zero. The isotherms for the
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)