SOLVENT SKIN INTERACTIONS 329 The polypropylene membrane (Celgard 2400, Celanese Corp.) had an effective pore size of 0.02 •m and nominal thickness of 25 •m. Approximately one square inch pieces of the membrane were cut from a sheet 24 hours prior to the experiment. They were washed in 50 ml of ethyl alcohol to remove any chemical agents deposited on the surface. The washed and dried pieces were carefully weighed and soaked in mineral oil USP (Fisher Scientific). One-half hour before the experiment, each piece of membrane was removed from the oil. Excess oil was removed carefully by repeated wiping. The membrane was then accurately weighed and the amount of mineral oil retained was calculated. The weight gain ranged from 48 to 52 percent of the initial dry weight. Membranes were then cut to the required size and used for penetration studies. PENETRATION EXPERIMENTS The diffusion apparatus, procedure, and assay have been described (4). The receptor solution (normal saline) was maintained at 37 ø + 0.1øC. All experiments were performed at least in triplicate. Individual flux values for each donor solvent were averaged mean flux values are repQrted in this paper. RESULTS AND DISCUSSION BENZOCAINE SOLUBILITY Table I shows the measured values of benzocaine solubility in the three solvents studied. Values obtained for water and propylene glycol were in close agreement with those reported earlier (6-7). Table I Values for Benzocaine Permeation • Solubility at 30 ø (mg/ml) j, (mg cm -2.hr •) Polypropylene Hairless Mouse Skin Water 1.26 0.089 0.10 Propylene glycol 146 0.16 0.094 Polyethylene glycol 400 435 0.13 0.010 PENETRATION EXPERIMENTS Figure 1 is a typical plot of benzocaine penetration through the inert polypropylene membrane. The curves are linear, signifying steady state penetration. The steady state flux, J, for membrane controlled penetration under sink conditions is given by Eq. 1. J=KC (1) In this equation, K is the permeability constant, and C is the donor permeant concentration. An equivalent relationship, expressed in terms of different parameters, is given by Eq. 2 (8). J--La (2)
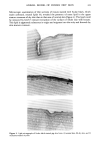
330 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS I0- 6 - 4 - 2 / I I i 3 5 Time,hr Figure 1. Benzocaine penetration through polypropylene membrane from aqueous solution, 1.26 mg/ml. Each curve represents a separate membrane. Here, a is the thermodynamic activity of permeant in the vehicle, and L is a constant. Provided that solvent-membrane interactions are absent, the value of L is independent of the solvent. To determine the influence of vehicle-membrane interactions, it is necessary to compare penetration behavior of the same substance from different solvents in such a way that the afiSnity of permeant for each solvent is compensated. Differences in flux must then be due to vehicle-membrane interactions, and the extent of deviation from the predicted value is a quantitative measure of the effect of such interactions on skin penetration. To evaluate a, we adopt the convention that the activity of pure solute is unity. In a saturated solution, dissolved permeant is in equilibrium with excess solid. All saturated solutions of the same substance therefore have an activity of unity. At the saturation concentration, the flux will be maximal. Denoting the concentration at saturation by Cs and the corresponding flux byJs and substituting in Eq. 1 yields Eq. 3. Js = K Cs (•) Solving for K and substituting in Eq. 1 produces Eq. 4. J = Js C/Cs (4) Equation 4 states that a plot of steady state flux vs. C/Cs should be a straight line passing through the origin. Figure 2 is a plot of mean flux against aqueous benzocaine concentration expressed in terms of Cs. The relationship is indeed linear with a zero intercept, in agreement with Eq. 4. Similar results were obtained for the other two solvents and the polypropylene membrane. However, it was not possible to work with saturated solutions in propylene glycol and polyethylene glycol 400 with the polypropylene membrane because of benzocaine precipitation in the donor. This was probably due to transport of water
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)