j. Soc. Cosmet. Chem., 41, 93-102 (March/April 1990) New odorous compounds derived from some terpenes OLCAY ANA0, NACIYE TALINLI, TOLAY MAZLUMO•LU, and OZKAN SEZER, Faculty of Science, Department of Chemistry, Technical University of Istanbul, Maslak 80626, Istanbul, Turkey. Received November 30, 1989. Synopsis Three terpene compounds, -•/-terpinene,13-pinene, and p-menth-l-ene were reacted with iodine-silver ace- tate in moist acetic acid (Woodward's method) to yield cis-diols. Reaction with dimethyldiazomalonate in the presence of Cu(II) acetylacetonate gave dimethoxycarbonyl derivatives. The isolated cis-diols were reacted with acetone in slightly acidic medium to prepare dimethylacetal derivatives. Acetal and dimethoxycarbonyl derivatives were found to possess different and finer odors than those of the starting materials. Comparative analysis of the odor of the acetals of the diols indicated the significant role of the gem-dialkyl group in the dioxolane ring for imparting pleasant, floral-balsamic or floral-woody odors to the products. It is known that the presence of an ester function in an organic compound generally causes odorous proper- ties. Furthermore, the results obtained by comparison of the odors of the inital terpenes and final gem- diester derivatives showed that a significant effect on the odor can be attributed to the existence of these new functional groups. INTRODUCTION The interest in (+)-3-carene and ot-pinene as intermediates for syntheses of pesticides and in odoriferous compounds and some other products has grown in recent years (1-2). Generally, most reactive sites of terpenes are their double bonds and the allylic C-H positions. Several studies have been made on the addition reactions to the double bonds of terpenes, especially of (+)-3-carene (3-4). In those studies, transformations of the double bonds to vic-diols by several reagents were investigated. The diol synthesis, which seems quite easy, involves great problems. Different stereo- isomeric diols have been obtained, depending on the positions of the substituents and the kind of hydroxylation agents employed (5). In many undergraduate organic text- books, a suggested method of cis-dihydroxylation is oxidation of double bonds by KMnO 4. However, its synthetic utility is limited because the diol or other groups in the molecule may oxidize further (6). Other methods to achieve cis-diols are OsO4 oxidation and Woodward's procedure, which involves reaction of an olefin with iodine and silver acetate in moist acetic acid. The former is both expensive and toxic, also 93
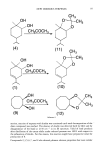
94 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS showing no significant increase in yields compared with the latter (5). Therefore, in the present work, Woodward's method was chosen for the production of cis-diols (Scheme 1). On the other hand, the carbene/carbenoid reactions, especially with compounds having multiple bonds, have been an important research area for organic chemists in recent years (7-11). As it is known, reactions of diazo compounds, being mainly carbene precursors with various olefins, have been studied under photochemical, thermal, and catalytical conditions, yielding addition, insertion, and carbene dimerization products (12-13). The overall reaction is summarized in Scheme 2. RESULTS AND DISCUSSION In the present work, the above-mentioned reactions of three terpene compounds, terpinene, p-menth-l-ene, and •-pinene, which were raw materials isolated from several essential oils (18-22), were investigated (Scheme 3). One of the problems was the isolation in high purity of the expected products from the crude reaction mixture. As described in the Experimental section, only a few reactions, purification by crystalliza- tion could be achieved. The preparative thin-layer chromatography technique (p-TLC) was generally used to separate and purify the p.roducts for identification. The weight percentages of individual compounds relative to the total product weights (from GLC) are summarized in Table I. Hydroxylation of 'y-terpinene by the procedure described in the Experimental section gave only one kind of cis-diol 1, the structure of which was identified by its tH-NMR spectrum: Methyl protons resonated at 1.22 ppm, whereas the signal of the same protons appeared at 1.63 ppm in 'y-terpinene, proving that only the methyl-substituted double bond had reacted. The signal at 5.31 ppm could be attributed to the olefinic proton. These data showed the greater steric hindrance around the isopropyl-substituted double bond. Absorbdon of six methyl protons of the isopropyl group took place as a doublet centered at 1.0 ppm, and the proton attached to the hydroxylareal carbon ab- sorbed at 3.64 ppm as doublet-doublet and the other protons of the ring between 1.9-2.18 ppm, whereas the two OH protons resonated at 2.25 and 2.30 ppm, respec- tively. All rates of integrations were in good agreement with the suggested structure of 1. Expected product was also obtained from p-menth-l-ene by the same procedure. But the stereochemistry of compounds 1 and 4 could not be determined. Application of Scheme !
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)