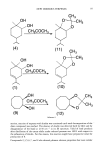
NEW ODOROUS TERPENES 97 CH3 -CH a OH CH3COCH• • OH (4) OH HaCOCHa , (1) (1 1 ) CH 3 O--•-C O (10) H3 • "•O H (9) Scheme 4 ..O CH 3 (12) section, reaction of terpenes with dmdm was continued until total decomposition of th 'e diazo compound was reached. The absence of dmdm was detected both by GLC and by disappearance of the band at 2130 cm-• in its IR spectrum. Yield of total products after distillation of the excess olefin under reduced pressure was 100% with respect to the exhaustion of dmdm. For this reason, this reaction might have preparative value for production of 8. Compounds 2,3,5,6,7, and 8 also showed pleasant odorous properties that were milder
98 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table II Conversion Ratios of Dials to Acetals (GLC) Dial Acetal reactions (%) (1) 98 (4) 97 (9) 99 and finer than those of the starting reagents. We conclude that the introduction of gem-diester functions into these terpenes provides several new compounds possessing odor properties that could be of use to the fragrance industry. EXPERIMENTAL SPECTROSCOPIC AND GAS CHROMATOGRAPHIC STUDIES Infrared spectra were measured on a Schimadzu IR-400 Model Spectrophotometer, and •H-NMR spectra were recorded by a Bruker 200 MHz Model instrument using TMS as CH2 addition allylic insertions allyl rearrangement ins. (8) CH 3 CH3 X2HC '-.% addition (8) allylic insertions X'CO2CH 3 /•OHX2 HaO'/•'-OX2H allyl rearrangement insertions Scheme 5
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)