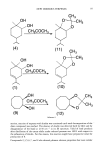
148 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS glucuronate HOO0• 0 . 5,ot-androst- 16-ene-3, [3-ol glucuronide androstenol 5, ot-androst- 16-ene-3, [3-ol sulfate % HOSO=- Figure 1. Hydrolysis of steroid conjugates. sulfate human axillary microbiota. Preliminary in-house studies demonstrated that axillary or- ganisms produce the enzymes, and there was some reason to think that organisms found in axillae of those who form little odor ("low" odor-formers) might be deficient in producing these enzymes. A double-blind experiment was run, therefore, on swabs taken from the axillae of twenty men who had been classified by professional odor evaluators (Hilltop Laboratories, Cincinnati, OH) as ten "high" and ten "low" odor formers. Elutions of the swabs were plated on Petri plates with culture medium con- taining fiuorogenic substrates for the suspected enzymes, and any liberated fluorescence
LETTERS TO THE EDITOR 149 was estimated under UV light. On plates with medium containing 4-methylumbelli- feryl glucuronide (substrate for beta-glucuronidase) (4), we found that nine out of ten "high" odor formers had beta-glucuronidase and that nine out of ten "low" odor formers had little or none (Table I) on plates with 4-methylumbelliferyl sulfate (substrate for aryl sulfatase), six out of eight of the "high"s had detectable aryl sulfatase activity (two samples were lost), and eight out of ten "1ow"s had little or none. Similar fiuorogenic substrates were used to detect the enzymes in flltrates of medium in which cultures of mixed axillary bacteria had been grown. The results of our experiments substantiated the hydrolytic theory completely, and the details will appear in a forthcoming paper. Edward Eigen REFERENCES (1) W. C. Noble and Somerville, Microbiology of Human Skin (Lloyd-Duke Medical Books, London, 1981), p. 93. (2) A. Nixon, A. I. Mallet, and D. B. Gower, Simultaneous quantification of five odorous steroids (16-androstenes) in the axillary hair of men, J. Steroid Biochem., 29, 505 (1988). (3) R. Hobkirk, Steroid sulfotransferases and steroid sulfate sulfatases: Characteristics and biological roles, Can. J. Biochem., 63, 1127 (1985). (4) R. W. Trapeta and S.C. Edberg, Methylumbelliferol-beta-D-glucuronide-based medium for rapid isolation and identification of Escherichia coli, J. Clinical Microbial., 19, 172 (1984). (5) S. Siegel, Nonparametric Statistics for the Behavioral Sciences (McGraw-Hill, New York, Toronto, London, 1956), p. 196. TO THE EDITOR: Hair waving technology is well established. The principal physicochemical changes taking place during waving have been ascertained, and a wide range of rationally de- rived formulations have been developed to meet demands of style and hair type. Excel- lent reviews of the state-of-art in this field can be found in two recent books by Robbins (1) and Zviak (2). There are occasions, however, where the waving result does not match the expectation of the consumer, and research continues to further elucidate and refine the mechanism of bond cleavage and re-formation. We wish to report here on some changes in fiber properties in the course of the waving process that might be of relevance to the final condition and appearance of hair. Our measurements were carried out on single fibers using a differential extension in- strument (3), which allows for continuous monitoring of fiber length while a chemical treatment is in progress. Another important feature is that fiber extensibility can also be assessed by intermittent application of a preselected fixed load that induces an instanta- neous elongation, referred to as the differential extension (DE). For short ranges of fiber extension, the DE represents the inverse of the fiber modulus. The graphic representa- tion of a measurement consists of a continuous recording of changes in fiber length with superimposed intermittent modulus determination.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)