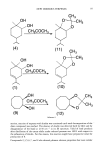
NEW ODOROUS TERPENES 101 Table III (continued) Compound % C, % H, and IR (cm-•) •H-NMR (CDC13) (8) (lO) 1,3,3-Trimethyl-7-iso- Calcd: 74.28 10.47 2920, 2850 propyl-2,4-dioxabi- Found: 74.49 10.36 1360, 1200 cyclo (4.3.0) non-7- C13H220 2 1140 erie (11) 1,3,3-Trimethyl-7-iso- Calcd: 73.58 11.32 2910, 2850 propyl-2,4-dioxabi- Found: 73.74 11.48 1360, 1200 cyclo (4.3.0) nonane C13H240 2 1140 (12) 3,3-Dimethyl-l,5-dioxa- Calcd: 69.76 11.63 2900, 2800 bicyclo (6.3.0) unde- Found: 69.72 11.84 1200, 1140 cane CloH200 2 5.44 (m, vinyl, 1H) 4.08 (dd, J: 2.8 and 7.6 Hz, 5-H, 1H) 0.98-1.02 (d, J: 7.08 Hz, 6H) 1.37 (s, acetalmethyl, 6H) 1.31 (s, 1-methyl, 3H) 1.9-1.6 (m, others, 4H) 3.90 (trip J: 5.8 Hz, 5-H, 1H) 1.46 (s, acetalmethyl, 3H) 1.38 (s, acetalmethyl, 3H), 1.31 (s, 1-methyl, 3H) 0.88 (trip, J: 7 Hz, 6H) 1.41-1.89 (m, others, 8H) 12 (m, 1-H and 5-H, 2H) 1.41 (s, acetalmethyl, 3H) 1.33 (s, acetalmethyl, 3H) 1.89-1.14 (m, others, 12H) GENERAL PROCEDURE OF ACETAL SYNTHESIS Diols (0.01 mol) were dissolved in anhydrous acetone (10 ml), and then p-toluenesul- phonic acid (0.01 g) was added and the mixture allowed to stand at room temperature for 24 hours. Then it was diluted with water, alkalized with 10% Na2CO 3 solution, and extracted several times with n-hexane. After drying over Na2804, the solvent was removed. Purity was controlled by GLC. Spectral data and elemental analysis are summarized in Table III. REFERENCES (1) M. Elliot and N. F. Janes, Synthetic pyrethroids--A new class of insecticide, Chem. Soc. Rev., 7, 473-505 (1978). (2) H. Sadowska and J. Gora, Synthesis and odor properties of carene and carane derivatives, Perfum. Flavorist, 7(1), 52-56 (1982). (3) W. Cocker and D. H. Grayson, The chemistry of terpenes. Part 23. Reaction of (+)-carane-3, 4- diol with sulphuric acid, J. Chem. $oc. Perkin Trans., I., 155-159 (1978). (4) A Henarich and Piatkowski, Stereochemistry of the carane system. Oxidation products of isomeric 3,4-carane diols obtained from (+)-3-carene, PolishJ. Chem., 58, 73-84 (1984). (5) E. Miiller, Houben-Weyl, Methoden der Organischen Chemie, Alkohole I 6/la/1 (Georg Thieme Verlag, Stuttgart, 1979), pp. 592-637. (6a) J. L. Simonsen, The constituents of Indian turpentine from pinus longifolia, roxb. Part I, J. Chem. $oc., 117, 570-574 (1920). (6b) P. P. Pillay and J. L. Simonsen, The constituents of Indian turpentine from pinus longifolia, roxb. Part IV, J, Chem. Soc., 359-364 (1928). (7) M. Jones and R. A. Moss, Carbenes (Wiley Interscience, New York, 1973). (8) N. S. Isaacs, "Reactive Intermediates," in Organic Chemistry (Wiley Interscience, New York, 1974), pp. 375-406.
102 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (9) M. Jones and R. A. Moss, Reactive Intermediates (Wiley Interscience, New York, 1978), p. 1. (10) W. Kirmse, Carbene Chemistry, Organic Chemistry (Academic Press, New York, 1971). (11) E. Miiller, Houben-Weyl, Methoden der Organischen Chemie, Photochemie II, 4/5b (Georg Thieme Verlag, Stuttgart, 1975), pp. 1158-1254. (12) D. S. Wulfman, N. v. Thinh, R. S. McDaniel, and B. W. Peace, Metal salt-catalyzed carbenoids, Part IX, J. Chem. $oc. Dalton Trans., 522-529 (1975). (13) D. S. Wulfman, Metal salt-catalyzed carbenoids--XII, Tetrahedron, 32, 1231-1240 (1976). (14a) D. S. Wulfman, R. S. McDaniel, and B. W. Peace, Metal salt-catalyzed carbenoids-XIII, Tetrahe- dron, 32, 1241-1255 (1976). (14b) D. S. Wulfman, R. S. McDaniel, and B. W. Peace, Metal salt-catalyzed carbenoids--XIV, Tetra- hedron, 32, 1251-1256 (1976). (15) D. $. Wulfman, B. G. McGibboney, E. K. $teffen, N.v. Thinh, R. $. McDaniel, and B. W. Peace, Metal salt-catalyzed carbenoids--XV, Tetrahedron, 32, 1257-1265 (1976). (16) O. Ana½ and C. Aydo•an, The catalyzed decompositions of some olefins with dimethyldiazomalonate, Chim. Acta Turcica, 10(22), 113-120 (1982). (17) O. Ana½, Some observations on the catalytic and photolytic reactions of cis cyclodecene and cis-bicyclo (3.3.0) oct-2-ene with dimethyldiazomalonate, Rev. Roum.de Chimie, 31(8), 787- 793 (1986). (18) E. Gildemeister, Fr. Hofman, Die Atherischen Ole IIIa (Akademie-Verlag, Berlin, 1960), pp. 70, 90, 164. (19) O. Ana½, Essential oil contents and chemical composition of Turkish laurel leaves, Perfum. Flavorist, 11, 73-75 (1986). (20) Analytical Methods Committee, Application of gas-liquid chromatography to the analysis of essential oils. Part XI: Monographs for seven essential oils, Analyst., 109, 1343-1360 (1984). (21) O. Ana½, and N. Talinli, The volatile leaf and berry oils from juniperus communis L. and juniperus oxycedrus L. having Turkish origin, Rev. Medicala, XXXIV(2), 167-171 (1988). (22) A. Hendrich and Piatkowski, Acetals and ketals of 3[3,413-carandiol--New odoriferous compounds from ( + )-3-carene, Perfum. Flavorist, 11, 85-88 (1986). (23) A. R. Pinder, The Chemistry of Terpenes (Chapman-Hall Ltd., London, 1960), p. 93.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)