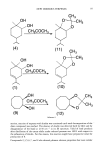
NEW ODOROUS TERPENES 95 R ,CHR'R" Catalytic ...: +N 2 - "(R') + ( ........ + , addition prod. insertion prds (allylic and others) R'R"CH--CHR'R" + R'R"C=CR'R" carbene dimers Scheme 2 Woodward's conditions to [3-pinene resulted in the formation of more than seven com- pounds via the cyclobutane ring opening, whereas the probable formation of the desired [3-pinene glycol could not be detected because of separation problems. As it is known, one of the characteristic reactions of cis-l,2-diols in slightly acidic medium is acetal formation with ketones. Therefore, the dimethylacetal derivatives of diols 1,4--and also 9, which was prepared--were synthesized, and the results are summarized in Scheme 4 and Table II. Compounds 10, 11, and 12 possessed odors which were different from and finer than those of the starting diols. The comparative analysis of the odor of the synthesized acetals of the investigated diols indicated the significant role of the gem-dialkyl group in the dioxolane ring in creating pleasant, floral-balsamic or floral-woody odors in the products. On the other hand, in the reaction of •/-terpinene with dimethyl-diazomalonate (dmdm), the GLC of the crude mixture showed a major component (71%). This compo- nent was extracted from p-TLC, and its 1H-NMR revealed two isomeric addition products (2 and 3) to the methyl-substituted double bond. Isomer ratio was 4: 1. Two different singlets were observed for the ester group of each isomer at 3.76, 3.63 for one, and 3.70, 3.59 for the other. Differences of the chemical shifts between individual singlets, approximately 22 Hz, were attributed to the effect of isopropyl-substituted double bond as proved by Dreiding models. In the reaction of p-menth-l-ene with dmdm, GLC revealed the presence of two main compounds (43.9 and 27.9%). Relatively pure samples of each component were ob- tained by p-TLC. The first one was a mixture of two isomeric addition compounds (5 and 6) having a ratio of 3:2 to each other from 1H-NMR data. The second compound was assigned the structure 7 as an allyl insertion product without specified stereochem- istry. In the last experiment, all possible products obtained in the reaction of dmdm with [3-pinene, excluding carben dimers and also ot-pinene, which could be isomerized from [3-pinene under present conditions, are illustrated in Scheme 5. Compound 8, which was detected as 89.1% by weight in the crude product, was extracted from p-TLC in a pure state. Its 1H-NMR spectrum showed clear signals at
96 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS H3C02c-•C• (2) H3C02C C02C C02CH 3 (3) i 3 OH (4) [• ': ,C (CO.• 3)2 •...,:,' ß • -. • CH •f'-I-(H3CO3)2H(0020'""'C P-MENTH-L-ENE /:-.... (5) /•-.....(6) (7) CH2CH(CO2CH3)2 b ,.•_ 13-PINENE (8) Scheme 3 a' 12/CH3COOAg/H20 b' dmdm/Cu(acac)2 5.29 ppm (vinyl P-), a neat triplet around 3.51 ppm (malonyl P-), and two dirtbrent singlets at 1.26 and 0.78 ppm (two methylsubstituents attached to the cyclobutane ring). Thus, the structure of 8 was easily identified. As mentioned in the Experimental Table I Proportional Contribution of Dials and Gem-Dimethaxycarbanyl Derivatives in Crude Products (GLC) Terpene Dial reactions % Carben reactions '•-Terpinene (1): 67.6 (2•'3) ß 71 R( + )-p-Menth- 1-ene (4): 65.2 (5•'6) ' 43.9(7):27.9 (IS)-(-) [•-pinene (8) ß 89.1
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)