146 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS 6. The octyl salicylate combinations were the most cost-effective. 7. The oxybenzone combinations were the least cost-effective. 8. The padimate O groups were all more cost-effective than the octyl methoxycinna- mate groups. REFERENCES (1) B. L. Diffy and J. Robson, A new substrate to measure sunscreen protection factors throughout the ultraviolet spectrum, J. $oc. Cosmet. Chem. 40, 127-133 (May/June 1989). (2) R. M. Sayre, P. P. Agin, G. J. Levee, and E. Marlowe, A comparison of in vitro and in vivo testing of sunscreen formulas, Photochem. Photobiol. 29, 559- 566 (1979). (3) C. Cole and R. VanFossen, Rapid in vitro evaluation of sunscreens: SPF and PFA, Photochem. Photo- biol., 47, 73S (1988). (4) L. Agrapidis-Paloympis, R. Nash, and N. Shaath, The effect of solvents on the ultraviolet absorbance of sunscreens. J. Soc. Cosmet. Chem., 38, 209-221 (July/August 1987). (5) H. Brown, Solubility of Suncreens in FINSOLV and other vehicles, Fintex technical bulletin (April 1989).
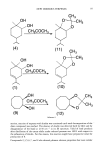
.]. Soc. Cosmet. Chem., 41, 147-154 (March/April 1990) Letters to the Editor TO THE EDITOR: This letter is written to outline a new mechanism for the generation of a significant component of body odor, steroidal axillary malodor. Various workers (1,2) have pro- vided evidence that the actual odorous substances in sweat are certain androstenols and androstenones, but it is not clear how skin bacteria produce them from their precursor steroids in apocrine secretions. Given that steroids, being generally water-insoluble, are typically transported and excreted in vivo as water-soluble conjugates such as sulfates or glucuronides, it seems likely that hydrolysis might well be the only contribution of the bacteria to odor formation (Figure 1). My colleagues at Coglate-Palmolive and I began experimental work on this idea in 1985, by looking for such enzymes as beta-glucuronidase and aryl sulfatase (3) in Table I Beta-Glucuronidase Activity in Axillary Sweat of "High" and "Low" Odor Formers Subject number Aryl sulfatase Beta-glucuronidase and group activity activity 2L + 3L - 4H - 5H + 6H - 7L - 8H + 9 H Lost 10 H + IlL - 12 H + 13 L - 14 H Lost 15 L - 16 H + 17 L + 18 H + 19 L - 20 L - Using the contingency coefficient as a measure of association (5), there is more than 99% probability of correspondence between "high" order formation and beta-glucuronidase activity, and more than 95 % prob- ability of correspondence between "high" order formation and aryl sulfatase activity. 147
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)