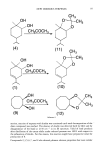
NEW ODOROUS TERPENES 99 an internal standard. Gas chromatogrophic studies were performed on a Schimadzu instrument, Model 5A, equipped with an F.I.D. detector. OV-17 3% on Chromosorb w 100/120 mesh, 3 mm. ID. X 3 m column was used. The following conditions were imposed: injection and detector temperature: 250ø carrier gas (N2): 55 ml/min tem- perature: isothermal at 165ø integrator: ITG-4A. Reproducibility of results was -T-0.5% under the same conditions. Internal standards were used for calculation. Three runs were carried out in each case. Elemental analyses were performed on Hereaeus Microstandard equipment. PREPARATIVE THIN-LAYER CHROMATOGRAPHY STUDIES (P-TLC) P-TLC studies were carried out using Silicagel HF254 layers (1 mm). Solvents were CH2Cl•:CC14:n-hexane:ethylacetate (75:10:10:5) and iso-octane:iso-amylacetate (85:15). As detection spray (for pattern plate only), 3.5% ethanolic solution of phos- phomolybdic acid was used. INITIAL MATERIALS AND STANDARDS In our previous papers, we had synthesized some of the compounds related to this study (18-19). Dimethyldiazomalonate (starting from p-toluene sulphonylazide), carbene dimers (tetramethoxycarbonylethane and tetramethoxycarbonylethylene) were prepared by the method of Wulfman and co-workers. (IS)-(-) [3-pinene and 'y-terpinene (Fluka) were ca. 95%, and R(•-)-p-menth-l-ene (Fluka) was 99% in purity. GENERAL PROCEDURE FOR THE REACTIONS WITH dmdm A solution of 0.07 mol of Cu(acac) 2 in 0. 125 mol olefin was refluxed, while a solution of 0.01 mol of dmdm in olefin (0. 125 mol) was added dropwise (ca. six drops per minute). After completion of dmdm addition, samples taken from the continuing reac- tion at time intervals were analyzed by GLC and IR in order to ensure the end of the reaction. The mixture was filtered while hot, and the excess olefin was recovered by vacuum distillation. The residue was analyzed by GLC and p-TLC. The zones that could be extracted from p-TLC were recontrolled by GLC to ensure their purities, and only pure fractions were identified by IR, 1H-NMR, and elemental analysis. GENERAL PROCEDURE FOR THE SYNTHESIS OF Cis-DIOLS While olefin (0.08 mol), glacial acetic acid (320 ml), and silver acetate (0.2 mol) were vigorously stirred at room temperature, iodine (0.17 mol) was added portionwise for over 1.5 hours. Water (1 ml) was then added to the reaction mixture, which was heated for 4 hours on a water bath while being stirred. AgI was filtered off after cooling the reaction mixture, and the tiltrate was evaporated in vacuo to remove acetic acid and unreacted olefin. The residue was hydrolyzed by heating with 10% ethanolic KOH solution for one hour (pH 11.5). The resulting mixture was diluted with water and extracted successively with n-hexane-ether (1:1) mixture. The solvent was removed and diols were crystallized from n-heptane.
100 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Table III Spectral Data and Elemental Analysis of Products Compound % C, % H, and IR (cm-•) •H-NMR (CDC13) (8) (2?3) and lot-Methyl-4- Calcd: 67.66 8.29 3050, 2970 isopropyl-7,7-di- Found: 67.70 8.30 2890, 1750 methoxy carbonylbi- C•3H2204 1740, 1620 cyclo (4.1.0) hept-3- 1440, 1025 ene (5?6) and lot-Methyl,4- isopropyl-7,7-di- methoxy carbonylbi- cyclo (4.1.0) Heptane (7) 6-Dimethylmalonyl-(R)- p-meth-l-ene (8) 10-Dimethylmalonyl- ( 1S)-ot-pinene (1) p-Menth-4-en-cis- 1,2- diol (4) p-Menthane-cis- 1,2-diol (9) Cyclooctan-cis- 1,2-diol Calcd: 67.16 8.95 2970, 2885 Found: 67.36 8.90 1740, 1460 C15H2404 1440, 1020 Calcd: 67.16 8.95 3015, 2975 Found: 67.38, 8.90 2875, 1770 C15H240 4 1740, 1640 1450, 1440 Calcd: 67.66 8.27 3100, 3000 Found: 67.72 8.42 2970, 2920 C15H220 4 2850, 1740 1650, 1440 Calcd: 70.58, 10.58 3400, 2960 Found: 70.70 10.42 2900, 1670 C•oH•302 m.p. 63 ø 1360, 1050 Calcd: 69.76 11.62 Found: 69.90 11.61 CloH2oO2 m.p. 74øC Calcd: 66.66, 11.11 Found: 66.78 11.14 C8H1602 m.p. 76øC 3425, 2925 286O, 1360 34OO, 325O 29OO, 285O 1050 5.52 and 5.13 (m, vinyl, 1H) 3.70 and 3.76 (s, ester, 3H) 3.63 and 3.59 (s, ester, 3H) 2.70 and 2.41 (m, allylic, 2H) 2.53 and 2.30 (m, allylic, 2H) 1.25 and 1.29 (s, methy, 3H) 1.00 and 0.93 (dd, J: 1.4, J: 6.99 and J: 1.8, J: 6.35 Hz, 6H) 3.70 (s, ester, 6H) 0.83 (s, methyl, 3H) 0.825 (d, J: 6.67 hz, 6H) 1.00-2.2 (m, 9H) and 3.73 (s, ester, 6H) 0.83 (s, methyl, 3H) 0.817 (d, J: 6.69 hz, 6H) 1.00-2.2 (m, 9H) 51 (m, vinyl, 1H) 3.736 (s, ester, 3H) 3.73 (s, ester, 3H) 3.55 (d, J: 8.47 Hz, malonyl, 1H) 2.89 (m, substituted allyl, 1H) 1.61 (s, methyl, 3H) 0.85 (d, J: 6.4 Hz, 6H) 1.00-2.00 (m, 6H) 5.29 (m, vinyl, 1H) 3.72 (s, ester, 6H) 3.51 (t, J: 7.6 Hz, mal- onyl, 1H) 2.57 (dd, J: 7.7 and 1.5 Hz, substituted allylic, 2H) 1.26 (s, methyl, 3H) 0.78 (s, methyl, 3H) 1.07-2.09 (m, 3H) 5.31 (m, vinyl, 1H) 3.64 (dd, J: 11.2 and 5.6 Hz, 1H) 2.25 (s, OH, 1H) 2.30 (s, OH, 1H) 1.22 (s, methyl, 3H) 1.00 (d, J: 6.5 Hz, 6H) 1.9-2.18 (m, others) 3.35 (dd, J: 10.8 and 4.5 Hz, 1H) 1.90 (s, 20H, 2H) 1.26 (s, methyl, 3H) 0.88 (d, J: 7 Hz, 6H) 1.17-1.84 (m, 7H) 3.29 (d, J: 8.3 Hz, 2H) 2.28 (s, 20H, 2H) 2.0-1.52 (m, 12H)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)