CLEANING HAIR 345 portant to consumers, also need to be optimized. Related to this, the efficiency of the surfactant for cleaning other soils from hair must also be considered in conjunction with the proposed market (mature, developing, etc.) where the shampoo is to be sold. REFERENCES (1) J. Clarke, C. R. Robbins, and B. Schroff, Selective removal of sebum components from hair by surfactants, J. Soc. Cosmet. Chem., 40, 309-320 (1989). (2) D. Thompson, C. Lemaster, R. Allen, and J. Whittam, Evaluation of relative shampoo detergency, J. Soc. Cosmet. Chem. 36, 271-286 (1985). (3) W. G. Spangler, H. D. Cross, and B. R. Schaafsma, A laboratory method for testing laundry products for detergency, J. Amer. Oil Chem. Soc., 42, 723-727 (1965). (4) M. J. Rosen, Surfactants and Interfacial Phenomena, 2nd ed. (Wiley-Interscience, 1989), pp. 363-392.
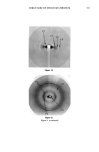
j. Soc. Cosmet. Chem., 41, 347-358 (November/December 1990) Study of lipid and non-lipid structure in human stratum corneum by X-ray diffraction J. c. GARSON, J. DOUCET, G. TSOUCARIS, and J. L. L•V•QUE, Laboratoires de Recherche de L'Oreal, Aulnay sous Bois (J.C.G., J.L.L.), LURE, Bat. 209 C, Universitg de Paris Sud, Orsay (J, D. ), and CNRS, UPR, Universitg de Paris Sud, Chatenay Malabry ( G. T, ), France Received September 21, 1990. Presented at the IFSCC Meeting, New York, October 1990. Synopsis The molecular organization of human stratum corneum (SC) is still poorly known, in spite of its importance for the understanding of its biological functions. By using ø synchrotron X-ray diffraction and oriented samples, new basic features were revealed in the 2.5-to-125 A domain. The X-ray patterns were richer and more complex than previously published. Lipid extraction allows discrimination between the proteic or lipidic origin of the numerous arcs and rings observed. Concerning the proteins, we found that the keratin organization (or versus {3) in human stratum corneum depends on its origin and environment, contrary to the commonly accepted ot form for skin keratin. For common SC it would be close to {3, and for "callus" type it is or. An unknown {3-pleated sheet protein with an 9.4 • interchain distance was inferred from the diffraction data. Reticular distances of the lipidic lameliar organization were evidenced by small-angle diffraction (10-65 •). Wide angle diffraction (2.5-10 •) showed intermolecular distances of different species of lipids. Certain features of the X-ray diagrams can be assigned (i.e., cholesterol). Liposomes, which are made also of organized lipids, can be characterized by an X-ray diagram. This fact is important for cosmetic research, providing the possibility to study the interaction between the stratum corneum and liposomes. Some examples will be reported. INTRODUCTION X-ray diffraction is the method of choice for the study of the spatial organization of macromolecules in biological tissues. This technique has been used for a large number of years in the investigation of atomic structure in metals and alloys and is a basic research tool in crystallography. In the field of biology, interest in X-ray diffraction increased when Watson and Crick discovered the double-helix form of DNA for which they were awarded the Nobel prize. Prior to this work, the diffraction patterns of keratins obtained in 1933 by Astbury marked the beginnings of molecular biology. 347
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)