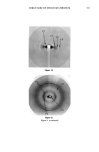
SURFACE DEPOSITS ON HAIR 381 The sodium laureth sulfate has an EO number of 2. The keratin hydrolysate is CTFA "hydrolyzed animal keratin." FIBER TREATMENT Fibers were mounted on stainless steel tabs and treated for 5 min at 30øC in a gently stirred solution. They were rinsed five times, 1 min each, with 200 ml demineralized water. After conditioning the fibers overnight at 65% RH and 2 IøC, a small segment of each fiber was removed for determining a receding contact angle O• by immersion wettability (6), and the rest of the fiber was scanned using the liquid membrane wetta- bility method (1). To assess the buildup of deposits on the fiber surface, the treatment was repeated three times the two levels of treatment are indicated by 1 x and 4 x in the figures and tables. Fiber perimeters were calculated from major and minor axes determined by microscopy. LIQUID MEMBRANE WETTABILITY SCANNING Our liquid membrane wettability scanning procedure has been discussed in a previous publication (1). The experimental arrangement is diagrammed in Figure 1. As the liquid membrane moves up the fiber, forces are generated at the two liquid surfaces, advancing and receding the net force is given by F = P'yLv(COS 0 a -- COS 0•), where P is the fiber perimeter, 'Yrv the surface tension of the membrane liquid, and and O• the contact angles in the advancing and receding modes. Assuming that cos determined in the separate immersion experiment, does not vary along the fiber permits calculation of cos 0•, a measure of surface energy. In these studies we used demineral- ized water as the membrane liquid. Although the assumption that equilibrium wetting PHOTOMULTIPLIER I X-Y RECORDER FILTER{360-IOOnm} . ¾ j COMPUTER ] Figure 2. Diagram of the Leitz MPV 1.1 microspectrophotometer with the PLOEMOPAK attachment for fluorescence measurements.
382 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS conditions exist is an oversimplification at the high scanning speeds used, our experi- ence has been that we can still achieve our objective of establishing dif)•rences in hair fiber surface energy. MICROFLUOROMETRY In applying microfluorometry to assess deposit thickness, a suitable fluorescent tracer is incorporated into the formulation either prior to deposition or as an aftertreatment. Interactions between the fluorescent probe and formulation components result in in- creased fluorescence, presumably because changes in the microenvironment of the tracer molecule cause an increase in its quantum efficiency. Assuming that film thickness is directly proportional to fluorescence intensity permits one to determine a relative film thickness distribution we have tested this assumption for a number of compounds and have found it to be correct. The sodium salt of fluorescein (CIAY-73), also known as uranine, proved an N, COONa Scheme. Uranine Na salt of fluorescein (CIAY-73). excellent tracer for the formulations investigated. Aqueous solutions of uranine excited at 450 to 490 nm emit brilliant green fluorescence at 540 nm. Uranine (0.1%) was incorporated into the various formulations, and hair fibers 5-cm long were immersed in these solutions for 5 min at 30øC, either once or five times, followed by one or five 1-min rinses with water at 30øC. After five applications, each with five rinses, some fibers were retagged with 0.1% uranine solution and rinsed once in water (both proce- dures for 5 min at 30øC). To evaluate the treated samples, we used a Leitz MPV 1.1 microspectrophotometer, with a Ploemopak fluorescence illuminator attachment, schematically shown in Figure 2. The distribution of deposits along the filament is characterized by monitoring fluo- rescence emission intensity as the scanning stage moves the specimen through the beam circumscribed by a variable measuring diaphragm. In these studies we used a 5-1•m x 5-1•m beam focused on the dome of the fiber and scanned 1.35 mm of fiber at 18 I•m/s. RESULTS AND DISCUSSION WETTABILITY SCANNING Figures 3a and 3b show wettability scans along the length of unoxidized and oxidized hair fiber specimens, respectively, and after one and four treatments with the formula-
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)