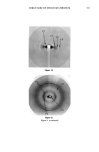
356 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS Chol. (1) •' Control Tr Treated • Chol. (2) Unknown compone I I I I I I I 50 40 30 20 15 10 9 'r RETICULAR DISTANCE (.•.) Figure 5. Densitometric analysis of two patterns: liposome-treated stratum corneum and control. SC, this method had previously given only relatively poor quality images, as resolution was limited by the intensity of the X-ray beams on the one hand, and the low degree of crystallinity of the structure on the other. The use of the synchrotron overcomes these problems and the results presented here indicate the future importance of this technique for understanding not only the structure and function of the SC, but also the action of physicochemical systems used in modern day cosmetology. With regard to the first point, these results provide two new findings and confirm a previous report: The new findings concern our knowledge of the protein constituents of the SC. Contrary to what has been accepted for over 50 years, intercellular keratin in the normal SC would not appear to be in o• form. The main result leading to this interpretation is the total absence of any band at 5.1 • that corresponds to the pitch of the ot helix. Nonetheless, on the very dry SC of the callus of the hand, this band is clearly visible, suggesting that the keratinization process defines the supramolecular architecture of this protein this would have direct consequences on the binding of water molecules to the SC. Further studies of pathologic SC should provide new information in this field.
STRUCTURE OF STRATUM CORNEUM 357 The second finding concerns the existence within the SC of a protein, the structure of which would also be close to a [3 structure. This unidentified protein might correspond to what microscopists call "desmosome remnants," which play, in addition to lipids, a fundamental role in intercorneocyte cohesion. The isolation and biochemical character- ization of this protein would be an important step toward understanding the desquama- tion process in the SC. With regard to SC lipids, numerous diffraction patterns were obtained and their inter- pretation is still underway. It is alreadoY clear, however, that the two rings situated, respectively, in the zones (63 -+ 3) A and (45 -+ 3) •, according to the samples studied, correspond to wide and narrow lipid bilayers in the intercellular spaces and are therefore of great interest in understanding the barrier function of the SC and its modi- fications under the influence of physical (UV, heat) and chemical treatment. Finally, we have shown the interest of this technique in characterizing vesicular systems and their various interactions with the SC. The stratum corneum is the anatomic struc- ture in the skin to which cosmetic agents are applied and also where they are designed to act. Detailed information on this extremely thin layer and its complex organization is necessary for a clear understanding of the relationship beween its structure and func- tion. In the same way, it is only through a precise knowledge of present-day physico- chemical systems, which are becoming more and more complex, that their interaction with the SC and their influence on its properties can be predicted. In both cases, studies based on X-ray diffraction using the synchrotron will take an increasingly important place. REFERENCES (1) G. Swanbeck, Macromolecular organization of epidermal keratin, Acta Dermato. Venereol. 39 (Suppl. 43), 5-37 (1959). (2) H. P. Baden and L. Bonar, The (x-fibrous proteins of epidermis, J. Invest. Dermatol., 51, 478-483 (1968). (3) H. P. Baden and A.M. Gifford, Isometric contraction of epidermis and stratum corneum with heating, J. Invest. Dermatol., 54, 298-303 (1970). (4) G. L. Wilkes, A-L Nguyen, and R. Wildnauer, Thermal stability of the cristalline lipid structure as studied by X-ray diffraction and differential thermal analysis, Blochim. Biophys, Acta, 304, 267-275 (1973). (5) S. E. Friberg and D. W. Osborne, X-ray diffraction study of human stratum corneum,J. Soc. Cosmet. Chem., 36, 349-354 (1985). (6) S. H. White, D. Mirejovsky, and G.I. King, Structure of lamellar lipid domains and corneocyte envelopes of murine stratum corneum. An X-ray diffraction study, Biochemistry, 27, 3725-3732 (1988). (7) P.M. Elias, Epidermal lipids, barrier function, and desquamation, J, Invest. Dermatol., 80, 44-49 (1983). (8) D.C. Swartzendruber, P. W. Wertz, D. J. Kitko, C. Kathi, M.D. Madison, and D. T. Downing, Molecular models of the intercellular lipid lamellae in mammalian stratum comeurn, J. Invest. Der- matol., 92, 251-257 (1989). (9) R. O. Potts, "Physical Characterization of the Stratum Corneum: The Relationship of Mechanical and Barrier Properties to Lipid and Protein Structure," in Transdermal Drug Delivery, Vol. 35 (Marcel Dekker, 1989). (10) W. P. Smith, M. S. Christensen, S. Nacht, and E. H. Gance, Effect oflipids on the aggregation and permeability of human stratum corneum, J. Invest. Dermatol., 78, 7-11 (1982). (11) J. L. L•v&que, M. Escoubez, and L. Rasseneur, Water keratin interaction in human stratum corneum, Bioeng. Skin, 3, 227-242 (1987).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)