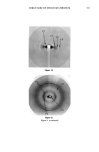
348 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS With regard to the skin, the quality of the patterns obtained in the stratum corneum (SC), for example, has been rather poor, mainly due to the fact that this keratinized membrane is of a composite nature, possessing both amorphous and crystalline zones that confer a poorly organized pseudosolid character. In addition, in order to obtain reasonably clear diffraction patterns in the SC, high doses of X-rays are used, requiring very long times of exposure if normal sources are used. However, certain studies of SC specimens of different types have been conducted re- cently and concern the organization of keratin (1-3) and lipids (4-6). So far, it has not been possible to determine whether keratin is organized in the ot or [3 form, due to the diversity of the SC specimens and the poor quality of the diffraction patterns obtained. With regard to lipid organization, apart from the results of White obtained in hairless mice, the patterns are very disappointing. They do, however, show an organization in bilayers, confirming the arrangements of polar lipids forwarded by Elias (7) and recently demonstrated by Swartzendruber (8). This form of lipid organization is of great interest, since according to numerous au- thors, it forms the basis of the barrier function of the stratum corneum (9). Lipids would also appear to be involved in the mutual adhesion of corneocytes and in the plasticity of the horny layer (10,11). We therefore studied the organization of proteins and lipids in the stratum corneum by means of X-ray diffraction, paying particular attention to the exact form (or or [3) of keratin in various SC specimens and the supramolecular organization of intercellular lipids. The observed intercellular lipid organization was compared with that found in liposome vesicles commonly used in cosmetics. In order to overcome the difficulties mentioned above (relative to the quality of the X-ray diffraction patterns obtained with the human SC), we used a high-energy X-ray source (synchrotron). MATERIALS AND METHODS EXPERIMENTAL SET-UP The X-ray beam was generated by the LURE synchrotron at the Universit• de Paris Sud. A simplified diagram of the equipment is given in Figure 1. In comparison with a conventional source, the intensity and resolution obtained can be increased by a factor of 100 and 5, respectively. A wavelength of 1.61 • was chosen. The specimens were placed on a goniometric head mounted on a multi-axis holder. A coincidental optical system enables the specimen to be oriented precisely relative to the beam axis. The distance between the specimen and the film can be varied in such a way as to obtain reticular planes from 2 to 70 •. Using another chamber, reticular planes from 20 to 200 • can be explored. The specimens placed in the chambers consist of a number of sheets of SC (2 to 16) that can be oriented either perpendicular or parallel to the beam axis. The SC sections (5 mm X 15 mm) are kept in place by means of specimen holders.
STRUCTURE OF STRATUM CORNEUM 349 Other experiment Linear accelerator •- e + Monochromator •nchrotron radiation Film SCHEMATIC OF THE EXPERIMENTAL SET-UP Figure 1. Schematic of the experimental set-up. SC SAMPLING The human SC specimens used were obtained during plastic surgery. The epidermis was separated from the dermis by treatment with humidified air at 56øC. The SC was separated from the epidermis by trypsin treatment. LIPOSOMES The vesicles studied were obtained by agitating a mixture of nonionic lipids, choles- terol, and dicethyl phosphate (47.5/47.5/5). Transmission electron micrographs of the vesicles were obtained after indirect staining. RESULTS AND DISCUSSION PROTEIN COMPONENTS The results concerning the supramolecular organization of the protein components in the SC are given in Table I and Figure 2. This preliminary identification was obtained by the delipidization of SC using a chloroform/methanol mixture and comparison with untreated specimens. As seen in Table I, three groups of reticular distances can be distinguished, corresponding to three different aspects (diffuse zone, sharp arc, and broad band). It is difficult to interpret the first group, P 1, since it is situated in a diffuse zone. The values obtained for this group nonetheless confirm those reported by White for SKH mouse SC. Group P2 corresponds to the fundamental and harmonics of a fine arc situated at 9.4 •. This sharp arc represents the organizatioon of a protein on a domain sufficiently large to attribute a size of approximately 3000 A. (Scherrer's formula is used to determine the
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)