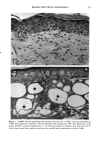
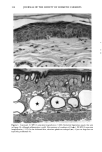
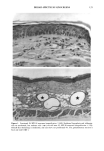
CHRONIC ACTINIC EXPOSURE ON LANGERHANS CELLS 119 were in good health with no record of abnormal reactions to sunlight. Before the project started, informed consent was obtained after the nature of the procedures had been fully explained. The subjects did not take any medication during the study. BIOPSY PROCEDURE A shave biopsy was taken from the inner aspect of the upper arm and the outer aspect of the forearm after local anesthesia with lidocaine. The inner aspect of the upper arm was considered to be a site without chronic sun exposure. In the main experiment, the sun-exposed site (outer aspect of forearm), biopsied during the winter and early spring, had not been exposed to sunlight within one month prior to biopsy in order to eliminate any acute effect of sunlight exposure. However, the sun exposure had not been con- trolled for the subject with photodamaged skin, and the biopsies were taken in the summer. EPIDERMAL SHEET PREPARATION The shave biopsy was immersed in 0.5 M NH4SCN tris-buffered saline for 20 minutes at 37øC. The epidermal sheet was separated from the dermis with fine forceps and rinsed with tris-buffered saline at pH 7.6. The epidermal sheet was then fixed in acetone for ten minutes and rehydrated in tris-buffered saline after fixation. The fixed tissue was immediately stained or held for up to three days in tris-buffered saline at 4øC prior to immunohistochemical staining. IMMUNOHISTOCHEMICAL STAINING Since HLA-DR and CDla markers are specific to Langerhans cells in human epidermis (17), we used mouse monoclonal anti-HLA-DR and anti-CDla antibodies as the primary antibodies, with a standard alkalinephosphatase technique as the staining method. Anti-HLA-DR antibody (DAKO Corporation, Carpinteria, CA) was used as a 1:60 dilution in tris-buffered saline, and anti-CDla antibody (Ortho Diagnostic Systems, Raritan, NJ, or DAKO Corporation) was used as a 1:5 dilution in DMEM with 10% bovine serum albumin. In the staining process the tissue was incubated with anti-HLA-DR antibody for 30 minutes at room temperature or with anti-CDla antibody for one hour at 37øC. The alkalinephosphatase-anti-alkalinephosphatase (APAAP) stain- ing was then performed using the DAKO Universal APAAP KIT System 40 TM. After a brief rinse in tris-buffered saline, the epidermal sheet was incubated with rabbit anti-mouse antibody. The tissue was then treated with the APAAP complex, and the color was developed using Fast Red solution as the chromogen (18). The tissue was mounted dermis side up on standard glass slides using a hydrophilic mounting medium (Glycergel TM, DAKO Corporation). Highly pigmented specimens were bleached by the following method if necessary: after staining, the tissue was immersed in 0.25% KMnO 4 solution at room temperature for ten minutes, rinsed in running water, then immersed in 5 % oxalic acid solution for 40 seconds, rinsed in running water, and placed in tris-buffered saline (pH 7.6) for ten minutes prior to mounting.
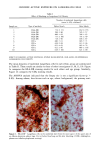
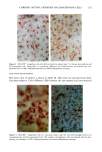
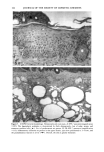
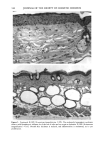
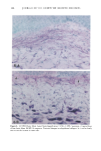
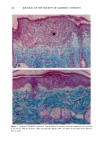
120 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS ENUMERATION Enumeration of Langerhans cells was performed by light microscopy with the aid of a calibrated eyepiece grid that defined a 250 x 250qxm field using a x40 objective and a x 10 ocular. As Langerhans cells are the only cells expressing CDIa or HLA-DR marker in the epidermis, all of the stained cells with dendritic morphology were counted. The number of cells per square millimeter was then calculated. Ten fields from each spec- imen were randomly chosen for enumeration, and the mean value was used as the density (cells/mm 2) of HLA-DR- or CDla-positive epidermal Langerhans cells. SKIN COLOR MEASUREMENT Skin color was measured with a tristimulus colorimeter (Model CR-200) Chromame- ter TM , Minolta Camera Co., Japan) to provide an indication of chronic sun exposure as expressed by the difference between the outer forearm and inner upper arm sites in both ethnic groups. Mean chromameter values for each subject were calculated from mea- surements taken at three randomly chosen sites from the outer forearm area and from the upper inner arm area. The L*a*b* system of measurement (CIE 1976) was used. The L* value measures brightness, and positive a* and b* values indicate redness and yellow- ness, respectively. STATISTICAL ANALYSIS For the validation of the bleaching method, a paired Student's t-test was used. The mean difference was considered significant when the p-value was less than 0.05. A four-way analysis of variance (ANOVA) incorporating factorial effects up to two-factor interac- tions was used for the chronic actinic exposure study. The factors were age, ethnic background, biopsy site (chronic actinic exposure), and primary antibody used for staining. Factors were considered significant when the p-value was less than 0.05. INSTITUTIONAL REVIEW BOARD APPROVAL Institutional Review Board approval was obtained prior to initiation of the study. RESULTS VALIDATION OF THE BLEACHING METHOD The bleaching method was validated by comparing the numbers of HLA-DR- and CDla-positive cells of nonpigmented skin before and after bleaching (Table I). There was no statistically significant difference between the numbers of cells before and after bleaching. Thus, the bleaching method did not affect the staining. Figures la and lb show HLA-DR-positive Langerhans cells in the epidermal sheet of an African-American subject before and after bleaching.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)