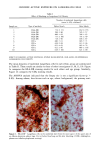
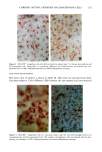
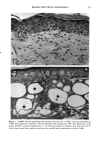
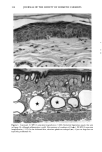
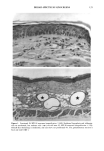
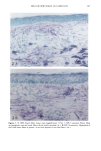
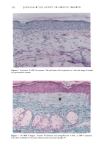
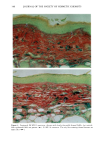
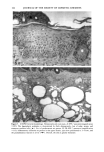
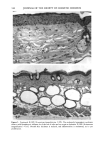
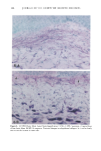
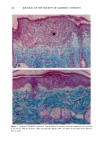
CHRONIC ACTINIC EXPOSURE ON LANGERHANS CELLS 125 epidermal Langerhans cells. Some of these data would superficially appear to differ from those reported by other investigators, but analysis of the study designs provides expla- nations to account for the observed differences. A reduction in Langerhans cell density at the sun-exposed sites ascribed to cumulative UV effects, reported by Thiers et al. (10) and Gilchrest et al. (11), was not observed in this study. The key factors that may account for the different results include our study of normal skin without evidence of actinic damage or recent sun exposure and the methods used for enumerating Langerhans cells. One of our criteria for subject selection was that the lower arms had not been exposed to sunlight for at least one month prior to biopsy, to eliminate acute UV effects. The study was performed during the late winter and early spring months, and the subject population consisted of women who had limited outdoor activity. The lack of recent sun exposure is important. Less than 3 MED of UV light exposure has been shown to result in a significant decrease in Langerhans cells within 24 hours (2,4). Further, we have shown that after approximately 2 MED of UV exposure, up to four or five weeks are required for recovery to baseline levels (20). Therefore, we had to evaluate the density of Langerhans cells without the effect of recent daily sunlight. However, past exposure to sunlight is necessary in this study in order to investigate the historical damage to Langerhans cells. Thus, skin color was measured to ensure the presence of past sun exposure. As a result, the presence of past chronic sun exposure at the dorsal forearm sites was suggested by the chromameter measurements. The dorsal forearm skin differed from the inner upper arm skin in its degree of darkness, and the similar AE values between the two sites for the two ethnic groups may suggest similar degrees of chronic sun exposure. Our finding of no significant difference between the sun-exposed and sun-protected sites in any of the subject groups is different from but not inconsistent with that of Thiers et al. (10), who reported that the number of Langerhans cells in sun-exposed skin is significantly less than that in sun-protected skin. The possible explanation is that their older subjects were from a patient group whose sun-exposed skin showed clinical evidence of solar damage, which might have had a secondary effect on the Langerhans cell density. In other words, the decreased Langerhans cell density of their patients may have been induced not by the direct effect of cumulative sun exposure, but by secondary effects from damaged dermal constituents that may interfere with the migration of Langerhans cells to the epidermis. This mechanism elucidates the discrepancy clearly since Lang- erhans cells migrate from bone marrow (21). In fact, we confirmed both decreased density and damaged morphology of Langerhans cells in clinically photodamaged skin by our staining method (Figure 4a-4b). Thus, our finding is not inconsistent with that of Thiers et al. (10). Gilchrest et al. also reported a difference between sun-exposed and sun-protected skin in subjects aged 46-68 (11). This study used preauricular skin, which is more likely to be exposed to doses of recent sunlight, for the sun-exposed site. Also, unstained cross sections of skin were used for enumeration this would not give a precise evaluation of cell density. From the four-way ANOVA, age, ethnic background, and the interaction between age and ethnic background were significant. These statistical conclusions are derived from the relatively small Langerhans cell density in the older African-American subject group. The Langerhans cell density in the older Caucasian group did not show such a decrease. This discrepancy constitutes one of the key points of our study. We think that this discrepancy is not due to the ethnic difference but rather is due to the difference in
126 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS mean age of these two groups. Gilhar et al. reported that the number of Langerhans cells in an aged group was approximately 50% of that in a younger group (13). Gilchrest et al. also reported a decrease of Langerhans cells in an aged group (4). A relationship between age-associated decrease of Langerhans cell density and deficiency in bone mar- row progenitors was suggested from the results of the transplantation of bone marrow cells in mice (13). Such a decrease would be reasonable in the light of generally decreasing immunological function with advancing age (21). However, our study did not show such a dramatic decrease but gave an equivocal result. The discrepancy between our study and Gilhar's study may be attributable to the difference in the age of the older age groups. The mean age of the older subject group in Gilhar's study was 82.1 years, which is considerably higher than that of our subject groups (Caucasian 66.0, African-American 69.6). On the other hand, the mean age of the older group in the report of Gilchrest et al. (4), 69, was almost the same as that of the African-American aged group in our study. In our study, the Langerhans cell density of the African-American elderly group is slightly lower than those of other groups. Thus, we consider that Langerhans cell density may start to decrease at around 70 years of age, and that the discrepancy between the two ethnic groups is not due to ethnic background but to the slight difference in the age of the two older groups. In other words, Langerhans cell density is maintained at the normal level until the eighth decade of life in healthy individuals, and the age of 66.0 is not old enough to show the decrease of Langerhans cell density. The primary antibody used for staining was also a statistically significant factor. This result most likely suggests that Langerhans cells in epidermis consist of two different subsets in phenotype, possibly a mature type and an immature type, but that these two subsets are considered to be of the same lineage. Harrist et al. reported that the number of CDla-positive Langerhans cells was approximately 2- to 3-fold the number of HLA- DR-positive Langerhans cells (22). Fithian et al. demonstrated that approximately 90% of CDla-positive cells were HLA-DR positive by immunofluoresence double staining (23). We cannot reach a definitive conclusion since double staining was not performed in our study. However, the number of HLA-DR positive cells was approximately 95% of that of CDla-positive cells. This result is comparable to that of Fithian et al. (23). Although two different subsets (CDla +/HLA-DR + and CDla +/HLA-DR-) may exist, our conclusion would not be affected since they showed the same tendencies. Our preliminary experiment showed that a small amount of acute UVB radiation, which simulated the sunlight exposure on a daily basis, differently damaged the Langerhans cell density of two ethnic groups possibly because of the protective effect of melanin pigmentation (data not shown). Therefore, before starting the study, we expected that the effect of chronic actinic exposure on Langerhans cell density might differ in these two groups. However, the effect of chronic actinic exposure was not apparent in this study. It is reasonable that there would not be any differences associated with ethnic back- ground if there is no chronic low-level UV effect. Our results indicate that Langerhans cell density, even at sun-exposed sites, is preserved as long as the systemic immune function is maintained in a normal condition, since Langerhans cells derive from bone marrow (24). We conclude that there is no identified chronic sunlight effect and no identified dif- ference between the two ethnic groups with respect to Langerhans cell density, and also
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)