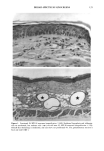
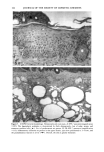
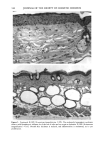
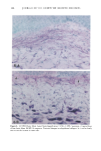
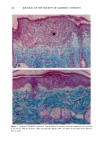
166 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS (13) M. Mao-Qiang, K. R. Feingold, C. R. Thornfeldt, and P.M. Elias, Optimization of physiological lipid mixtures for barrier repair, J. Invest. Dermatol., 106(5), 1096-1101 (1996). (14) R. Ghadially, B. E. Brown, S. M. Sequeira-Martin, K. R. Feingold, and P. M. Elias, The aged epidermal permeability barrier. Structural, functional, and lipid biochemical abnormalities in humans and a senescent murine model, J. Clin. Invest., 95(5), 2281-2290 (1995). (15) G. Imokawa and M. Hattori, A possible function of structural lipids in the water-holding properties of the stratum corneum, J. Invest. Dermatol., 84, 282-284 (1985). (16) G. Imokawa, S. Akasaki, M. Hattori, and N. Yoshizuka, Selective recovery of deranged water- holding properties by stratum corneum lipids, J. Invest. Dermatol., 87, 758-761 (1986). (17) G. Imokawa, S. Akasaki, Y. Minematsu, and M. Yawai, Importance of intercellular lipids in water- retention properties of the stratum corneum: Induction and recovery study of surfactant dry skin, Arch. Dermatol. Res., 281, 45-51 (1989). (18) G. Imokawa H. Kuno, and M. Kawai, Stratum corneum lipids serve as a bound-water modulator, J. Invest. DermatoL, 96, 845-851 (1991). (19) N. Marsh, P. M. Elias, and W. M. Holieran, Glucosylceramides stimulate murine epidermal hyper- proliferation, J. Clin. Invest., 95, 2903-2909 (1995). (20) P. Bowser and M. Gray, Sphingomyelinase in pig and human epidermis, J. Invest. Dermatol., 70, 331-335 (1978). (21) R. Freinkel and T. Traczyk, The phospholipases A of epidermis, J. Invest. Dermatol., 74, 169-173 (1980). (22) R. Freinkel and T. Traczyk, Acid hydrolases of the epidermis: Subcellular localization and relationship to cornification, J. Invest. Dermatol., 780, 441-446 (1983). (23) S. Grayson, A. G. Johnson-Winegar, B. U. Wintroub, et al., Lamellar body-enriched fractions from neonatal mice: Preparative techniques and partial characterization, J. Invest. Der matol., 85, 289-294 (1985). (24) H. Tagami and K. Yoshikuni, Interrelationship between water-barrier and reservoir functions of pathologic stratum corneum, Arch. Dermatol., 121, 642-645 (1985).
j. Sac. Cosmet. Chem., 47, 167-175 (May/June) A new cell culture method for phototoxicity testing' Effects of oral hypoglycemic and diuretic agents EDGAR SELVAAG, HELLE ANHOLT, JOHAN MOAN, and PER THUNE, Department of Dermatology, Ullevaal Hospital (E.So, P. T. ), and Department of Biophysics, Norwegian Radiumhospital, Montebello (H.A., J.M. ), University of Oslo, Oslo, Norway. Accepted for publication July 18, 1996. Synopsis Cervical cells from human in situ carcinoma, the NHIK (Norsk Hydro's Institute for Kreftforskning) 3025 cell, were utilized for screening phototoxic drugs. Sulphonamide oral antidiabetics and diuretics were incubated with the cells for one hour and then irradiated with broad-band UV and UVA. Cell death was dependent on UVA-fluence and drug concentration. Phototoxicity was demonstrated in the presence of two oral antidiabetics and ten diuretics. A number of substances, not previously described to exert phototoxic effects, were detected in this model. Electronmicroscopy showed that biomembranes were the main target, but nuclear damage was also de- monstrable. Addition of antioxidants decreased phototoxic cell death, indicating the participation of reactive oxygen species. This model proved not only useful in screening procedures, but also seems suitable for investigating mechanisms of action. INTRODUCTION It is important to identify possible photosensitizing agents before introduction into clinical therapy. A number of different test systems, ranging from red blood cells, yeast, and tissue cultures to animal models have been used to evaluate potential phototoxicity. Screening procedures are endorsed by the European Community countries (1). So far no in vitro or in vivo method has been able to recognize all known photosensitizers, and it is generally recommended to investigate several test models. The use of different light sources with varying emission spectra is only one of the factors responsible for conflicting results. On the other hand, the absorption spectra of the chromophors may change due to metabolism and binding to other components. Humidity, heat, and immunologic factors are other aspects that influence the results of phototoxicity in in vitro and in viva testing. Sulphonamide was introduced into clinical practice in the 1930s (2), and photosensi- tivity reactions due to sulphonamides were recognized shortly thereafter (3-5). By 167
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)