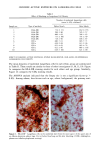
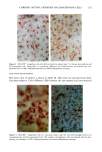
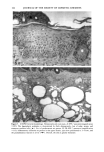
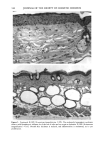
170 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS was kindly provided by Pharma Nord, Denmark. Ascorbic acid was dissolved in PBS (Dulbecco's phosphate-buffered saline) to a concentration of 0.5 mol/l, beta-carotene was dissolved in PBS buffer to a concentration of 0.025 mol/1, and alpha-tocopherol and ubiquinone were dissolved in DMSO to a concentration of 0.5 mol/1 and 0.25 mol/1, respectively. Of the antioxidant solutions, 5-20 p•l were added to 5 ml PBS buffer containing the test substance. The final concentration of the antioxidants added to the cell culture flask ranged from 0.025-2 mol/1. After incubation, the flasks were irradiated from above for 0-32 minutes (0-19.2 J/cm 2 UVA) with mylar-filtered irradiation from the "Bluelight 2000" apparatus or from below for 0-120 minutes (0-4 J/cm 2 UVA) with unfiltered irradiation from the UVA tubes. Control experiments with DMSO alone, DMSO and mylar-filtered light from the "Bluelight 2000" apparatus or UVA from the UVA tubes without photosensitizers, as well as irradiation with nonfiltered light from the "Bluelight 2000" apparatus, were performed. After irradiation 5 ml E2a medium with 30 per cent serum was added to the flasks, and the cells were incubated at 37øC for seven days. Then the cell colonies were fixed with absolute ethanol, stained with methylene blue, and counted. Cell survival was studied by measuring the colony-forming ability of the cells. Results are given as the median value of three experiments. For electronmicroscopic investigations, the test substance bemetizid in the 0.5 mM concentration was exposed to 2.5 J/cm 2 UVA from the "Bluelight 2000" apparatus. Five minutes after the end of irradiation, the cells were fixed with a mixture of 1% glutaraldehyde and 4% formaldehyde in 0.1 M cacodylate buffer, postfixed with cac- odylate-buffered 1% osmium tetroxide, and dehydrated in graded ethanols and propyl- ene oxide before being embedded in an Epon/Araldite mixture. Ultrathin sections were cut with diamond knives, floated onto 200-mesh copper grids covered by a supporting membrane, stained with uranyl acetate and lead citrate, and observed under a Philips CM-10 transmission electron microscope. RESULTS Phototoxic cell death was induced in the presence of the antidiabetic drugs glibencla- mide and gliquidone, and the diuretics bemetizide, bendroflumethiazide, benzylhydro- chlorothiazide, bumetanide, butizide, hydrochlorothiazide, hydroflumethiazide, piret- anide, polythiazide and trichlormethiazide after irradiation with the "Bluelight 2000" apparatus (Table I). Irradiation in the presence of the antidiabetics chlorpropamide, glipizide, glymidine, tolazamide, and tolbutamide and the diuretics chlortalidone, furosemide, indapamide, and xipamide did not induce a phototoxic reduction in the number of colony-forming cells. Irradiation with the UVA tubes induced phototoxic cell death in the presence of the antidiabetic glibenclamide and the diuretic bendroflume- thiazide, both in the concentration 0.5 mmol/1. Irradiation for one hour (2 J/cm 2 UVA) reduced the cell survival to about 1/40 in the presence of glibenclamide and to 1/10 in the presence of bendroflumethiazide. Two hours of irradiation (4 J/cm 2 UVA) com- pletely killed all the cells in the presence of both test substances. Addition of ascorbic acid and alpha-tocopherol reduced the phototoxic cell death in all the drugs investigated. The effects were dependent on the UV dose administered, the test substance concentration, and the antioxidant concentration. The inhibiting effects
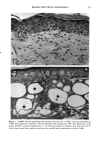
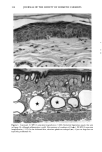
PHOTOTOXICITY TESTING 171 Table I Cell Survival (%) After Irradiation With the "Bluelight 2000" Apparatus in the Presence of Test Substances as Compared to Cell Survival in the Presence of Solvent Alone and After Irradiation with Mylar Filtered UV Light Irradiation with "Bluelight 2000" (min) Test substance (concentration) 0 1 2 4 8 16 32 Solvent (DMSO) and mylar- filtered UVA only 100 100 100 88 82 60 42 Glibenclamide (0.5 mmol/1) 100 100 74 63 0 0 0 Gliquidone (0.5 mmol/1) 100 100 77 63 0 0 0 Bemetizide (0.5 mmol/1) 100 53 49 11 0 0 0 Bemetizide (0.25 mmol/1) 100 53 49 11 0 0 0 Bendroflumethiazide (0.5 mmol/1) 100 58 29 0 0 0 0 Bendroflumethiazide (0.1 mmol/1) 100 96 63 12 0 0 0 Bendroflumethiazide (0.05 mmol/l) 100 74 88 61 35 0 Benzylhydrochlorothiazide (0.5 mmol/l) 100 67 56 28 0 0 0 Benzylhydrochlorothiazide (0.25 mmol/1) 100 67 56 28 0 0 0 Bumetanide (0.5 mmol/1) 100 100 75 56 19 0 0 Bumetanide (0.25 mmol/1) 100 96 82 60 53 0 0 Butizide (0.5 mmol/1) 100 100 42 21 0 0 0 Hydrochlorothiazide (0.5 mmol/1) 100 79 32 28 0 0 0 Hydroflumethiazide (0.5 mmol/1) 100 91 47 46 35 0 0 Hydroflumethiazide (0.25 mmol/l) 100 91 47 46 35 0 0 Piretanide (0.5 mmol/1) 100 100 67 70 12 0 0 Polythiazide (0.5 mmol/l) 100 51 19 9 0 0 0 Trichlormethiazide (0.5 mmol/1) 100 100 79 58 30 0 0 became more prominent when the irradiation time/dose exceeded 8 min/9.6 J/cm 2 UVA. No significant results were obtained with the addition of beta-carotene or ubiqui- none. When investigating the inhibiting effects of the antioxidants with the UVA tubes, no significant differences between the results with and without addition of antioxidants were recognized. The electronmicroscopical investigations showed destruc- tion of biomembranes, but nuclear damage was demonstrable as well. DISCUSSION Two out of seven oral antidiabetics and ten out of fourteen diuretics exhibited phototoxic action in our model. The absorption maxima of the test substances is in the UVA range at about 325 nm, which overlaps well with the emission maxima to the "Bluelight 2000" lamp. Not all potential photosensitizers revealed positive results in this model. This may be due to weak sensitizing capacity, but factors such as binding to, or penetration of, the
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)