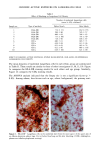
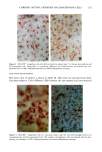
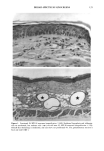
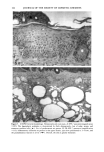
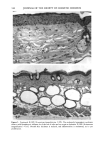
172 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS cellular membrane have to be evaluated. Another explanation for differences in photo- toxic response may be explained by the molecular structure of the substances. When comparing the phototoxic with the nonphototoxic drugs, it is noticeable that the phototoxic drugs all have either long lipophilic substitutes in the molecules (such as in glibenclamide, Figure 3a) or a double-ring structure (such as in bendroflumethiazide, Figure 3b). The nonphototoxic drugs have shorter lipophilic substitutes (such as in chlorpropamide, Figure 4a) or a one-ring molecular structure, so-called monosulpho- namides (such as in furosemide, Figure 4b). The more lipohilic substances may therefore induce phototoxicity more easily than the lesser lipophilic. Different subcellular targets also have to be considered. In the photohemolysis test, membrane damage is detected, whereas in more complex systems, DNA damage may play an important role as well. This is indicated in our electronmicroscopic investigations, where nuclear damage is demonstrable. This is in accordance with earlier findings showing thiazides to exert their main phototoxicity on biomembranes (15), but photomutagenic effects have also been reported (16). Photosensitizing substances seem to have one major target, but may induce damage to other sites as well (17). Matsuo and co-workers clearly demonstrated that the membrane lipids play an impor- tant role in thiazide phototoxicity (15). They irradiated red cells with longwave UV in the presence of the thiazide diuretics, and showed that the amount of peroxide was enhanced in deuterium and inhibited by antioxidants, indicating the involvement of oxygen radicals. The phototoxic properties due to our test substances were, in the same manner, clearly dependent on oxygen, as antioxidants, especially ascorbic acid, significantly inhibited the phototoxic action (18). The phototoxic properties of the test substances were only observed when test substances and cells were incubated together. Preirradiation of the test substances and subsequent incubation with the erythrocytes did not induce hemol- ysis. This may be explained by the involvement of oxygen radicals, which have a limited lifetime and diffusion length in cells (19). So far, two oral antidiabetics and eight diuretics have been investigated in vitro for phototoxicity (Table II). Johnson and co-workers demonstrated the photohemolytic effects of tolbutamide, but were unable to detect phototoxic properties in the presence I-I3OO 0=(2 Figure 3a. Molecular structure of glibenclamide. Figure 3b. Molecular structure of bendroflumethiazide.
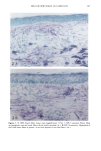
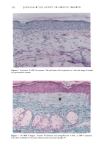
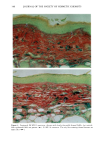
PHOTOTOXICITY TESTING 173 NH-- ((•2) 2-- (•{ 3 Figure 4a. Molecular structure of chlorpropamide. C Nil" (:I'I2 • Figure 4b. Molecular structure of furosemide. of furosemide and hydrochlorothiazide (20). Matsuo et al. induced phototoxic hemolysis in the presence of benzylhydrochlorothiazide, hydrochlorothiazide, methiclothiazide, and penflutizide (15). Using the Candida albicans test, tolbutamide (21), acetazolamide (22), chlorothiazide (21), and hydrochlorothiazide (20) have not shown a phototoxic growth inhibition. Using trichophyton mentagrophytes, Horio was able to demonstrate phototoxic growth inhibition with tolbutamide, whereas the tested diuretics chlorothi- azide, hydrochlorothiazide, methiclothiazide, and trichlormethiazide did not induce phototoxic effects (23). Morison and co-workers, utilizing the sensitive thymidin-uptake test, were able to induce a phototoxic decrease in the uptake of tritium-marked thy- midine into DNA in the presence of chlorpropamide and furosemide (24). Johnson et al. were not able to confirm their results with chlorpropamide, but demonstrated photo- toxic effects in the presence of hydrochlorothiazide (20). Finally, using tissue culture techniques with the HEp-2 cell line originating from a human laryngeal carcinoma, Freeman and co-workers demonstrated phototoxicity due to tolbutamide and hydrochlorothiazide among others (25). All these experiments in vitro were carried out with longwave UV irradiation. UVA irradiation to cultures of the human cervix carcinoma cell line NHIK 3025 induced phototoxic cell death in the presence of ten out of fourteen tested diuretics. Neither UV alone, nor the test substances, nor the solvent alone or in combination with the UV light reduced the colony-forming ability of the cells. Cell death was dependent on the UV dose applied and the test substance concentration. Irradiation from the "Bluelight 2000" apparatus induced significant phototoxic cell death after only one minute in the presence of the test substances bendroflumethiazide, bemetizide, and polythiazide. CONCLUSIONS The introduction of new drugs and chemicals demands methods to detect possible hazardous side effects. Screening for photosensitivity should be one of these precautions. Adverse reactions to electromagnetic irradiation, both acute and chronic, are well doc- umented. Our model, previously well-established in porphyrin research, seems valuable for in- vestigating phototoxicity, as two oral antidiabetics and eight diuretics, not previously detected in vitro as photosensitizers, were phototoxic in this assay. But not all known
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)