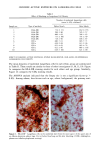
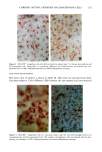
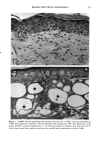
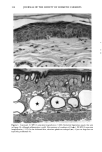
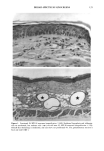
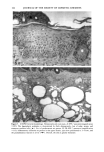
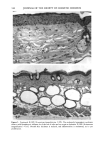
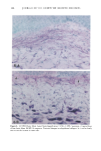
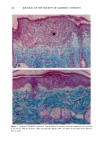
158 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS logical inhibitors of each synthetic pathway to alter barrier homeostasis (4-6), ascribable to specific lipid biochemical and membrane structural abnormalities in the stratum corneurn (4-7). Whereas these findings showed the separate requirement for each of the three key lipids for barrier homeostasis, previous studies also have demonstrated that maintenance of normal barrier function requires all three species of stratum corneum lipids together. Topical application of any one or two of the three key lipids delays or worsens barrier recovery following acetone-induced barrier disruption (8). In contrast, topical applica- tion of a lipid mixture, containing free fatty acid, cholesterol, and ceramide in an approximately equimolar ratio, allows normal barrier recovery (8). Moreover, optimal ratios of these three lipids accelerate barrier recovery following either acetone treatment or tape stripping, and some types of surfactant treatment of mouse skin, regardless of the extent of barrier disruption (9,10, 13). Because the large quantities of the ceramides needed to formulate such optimized mixtures may not be commercially available or affordable, we determined whether a naturally occurring, lipid-enriched mixture of animal origin, containing the three key lipids primarily as complex precursors, could enhance stratum corneum function. Our findings show that a natural lipid mixture, with an approximate lipid ratio of 1:1:3 (cholesterol:ceramides:fatty acids), accelerates barrier recovery following acute barrier disruption of murine and human skin. Moreover, this lipid mixture also enhances stratum corneum moisturization in both normal and damaged murine and human skin. METHODS AND MATERIALS MATERIALS Six- to eight-week-old male hairless mice were purchased from Simonsen Laboratories (Gilroy, CA) and fed Purina mouse diet and water ad libitum. Acetone and propylene glycol were purchased from Fisher Scientific (Fairlawn, N J). Cholesterol, ceramides, and palmitate were purchased from Sigma Chemical Company (St. Louis, MO). The natural lipid mixture, Y2, derived from animal porcine tissue, was purchased from Ocean Pharmaceutical (Weihai, P. R. China). LIPID BIOCHEMISTRY The lipid composition of Y2 was quantitated by high-performance thin-layer chroma- tography (HPTLC) followed by charring and scanning densitometery, as previously published (11). Briefly, 2-5 Ixg of the lipid extract was applied to the TLC plates for neutral lipid analysis, while 20 or 100 Ixg were utilized for polar lipid analysis. 0.2 to 1.0 Ixg of a polar lipid standard and 0.12 to 1.0 Ixg of a neutral lipid standard were applied to each side of the plates to generate standard curves, as well as to identify the major species. Neutral lipids were fractionated by developing the plates in petroleum ether:diethyl ether:acetic acid (80:20:1, vol), as described previously. Polar lipids were developed to 35 and 55 mm in chloroform:ethyl acetate:ethylmethylketone:2-propanol: ethanol:methanol:glacial acetic acid:hexyl acetate (34:4:4:6:20:28:4:1, vol), to 70 mm in chloroform:ethyl acetate:2-propanol:ethanol:methanol:H20 (46:4:4:6:28:6, vol), and then to the top of the plates in chloroform:methanol:acetone (80:10:10, vol). Sphin-
BARRIER FUNCTION AND HYDRATION 159 golipids were developed to 20 mm in chloroform:methanol:acetone:acetic acid (76:20: 4:1, vol), to 50 mm in chloroform:methanol:acetone (80:10:10, vol), and then to the top of the plates in chloroform:ethyl acetate:diethyl ether:methanol (76:20:6:2, vol). The plates then were scanned by scanning densitometry, and the lipid fractions were quantitated using CATS II software, as described previously (11). PROTOCOL FOR ANIMAL STUDIES Barrier function was assessed by measurement of transepidermal water loss (TEWL) with an electrolytic water analyzer (Meeco, Warrington, PA) (12). Barrier function was disrupted by repeated treatment with absolute acetone until TEWL rates exceeded 2.0 mg/cm2/hr. Immediately after treatment, TEWL was measured, and either 1.6% Y2 in propylene glycol:ethanol (7:3, v/v) or vehicle alone was applied to the treated areas (5-7 cm2). TEWL was measured at the time points indicated. Data are expressed as percent- age of recovery from time 0. For stratum corneum hydration studies, either 1.6% Y2 or 1-2% of synthetic lipids were applied to acetone-treated skin. Cholesterol, ceram- ides, and palmirate were applied individually or as a mixture at the final concentration of 1.1%, and the vehicle (propylene glycol:ethanol, 7:3, v/v) alone served as the control. Stratum corneum hydration, measured as capacitance, was assessed with a corneometer (CM-820, Courageq-Khazaka, Germany) both before and two hours after treatment. Data are expressed as percentage of change from levels immediately prior to the appli- cations of lipids or vehicle. PROTOCOL FOR HUMAN STUDIES In one group of twenty human volunteers (eight females, 12 males, ages 22 to 64 years), both forearms were treated with either acetone or tape stripping (Scotch type) until TEWL exceeded 1.0 g/m2/hr. Eighty microliters of vehicle was applied to the treated area of one arm, while the same volume of Y2 was applied at a concentration of 1.6% to the treated area (6-8 cm 2) of the other arm. TEWL was measured with an evapo- rimeter prior to and at indicated time points after vehicle or lipid applications. Stratum corneum hydration was assessed with a corneometer at the time indicated. In another group of 40 normal human volunteers (20 females, 20 males, ages 17 to 49 years) with no prior history of skin diseases, one shin was treated with the vehicle, while the opposite shin was treated with 1.6% Y2. Hydration studies in all subjects were per- formed two hours after application under comparable environmental condition and at the same time of the year (autumn). Statistical significances were determined using the Student's two-tailed T test. RESULTS LIPID COMPOSITION OF Y2 LIPID MIXTURE Previous studies have demonstrated that the ability of lipid mixtures to influence the barrier recovery rates is dependent on their composition. Therefore, we first analyzed the lipid composition of Y2. As shown in Table I, the total lipid content is 90.5% by total weight. The major lipids are glycosphingolipids (32%) and phospholipids (20.04%).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)