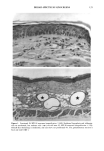
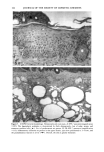
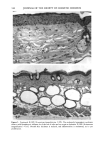
168 JOURNAL OF THE SOCIETY OF COSMETIC CHEMISTS chance, blood sugar-lowering and diuretic properties of oral sulphonamides were dis- covered, which led to the development of the oral antidiabetic drugs and the thiazides and loop diuretics (6). Clinical reports shortly after the introduction of these drugs showed photosensitizing properties with the antidiabetic (7) and diuretic derivatives (8,9) as well. In the industrialized countries, 16% of the population above 65 years suffers from diabetes mellitus and receive treatment with diet and oral antidiabetics (10). Similar prescription rates are found with the diuretics: in 1980 22.5 billion daily dosages were prescribed worldwide (11). Despite the extensive use of these substances, so far only two oral antidiabetics and eight diuretics have been investigated in vitro for phototoxic properties. The aim of this work was, on the one hand, to investigate the usefulness of the NHIK 3025 cell line, a model established in porphyrin research (12), in screening for photo- toxicity and mechanism studies, and on the other hand, to evaluate the potential phototoxic effects to a number of sulphonamide-derived substances. MATERIAL AND METHODS IRRADIATION SOURCES A broad-band UV irradiator ("Bluelight 2000" apparatus, H6nle, Martinsried, FRG) emitting 290-680 nm (Figure 1) and four fluorescent UVA tubes (Model 3026, Pho- tophysics, London, UK) emitting 340-420 nm, with an emission maximum at 405 nrn (Figure 2), were used in these studies. The emission spectra of the lamps was provided by H6nle and Photophysics, respectively. UVA and UVB fluence rates and fluence doses were measured with an integrating instrument (Centra-UV, Osram, Munich, FRG) E rel. I 250 270 290 310 330 350 370 390 410 430 450 Figure 1. Emission spectrum of the "Bluelight 2000" apparatus (H6nle, Martinsried, Germany) (1) and the transmission through the tissue culture flasks and mylar plastic (2).
PHOTOTOXICITY TESTING 169 E rel. ^ i i i i i • I s I $ I I I I I t I I I • I • I I I I I I I I 350 375 400 425 450 475 500 Figure 2. Emission spectrum of the four fluorescent UVA tubes (Model 3026, Photophysics, London, UK). equipped with two filtered photodiodes with different spectral sensitivities. A part of the UVB portion of the "Bluelight 2000" apparatus was absorbed by the tissue culture flasks. To eliminate the remaining main part of the UVB, a sheet of mylar plastic with a thickness of 36 }xm was placed between the light source and the tissue culture flasks. The transmission of UV through the tissue culture flasks and mylar plastic was deter- mined with a spectrophotometer (UV/visible spectrophotometer, Perkin-Elmer, Oslo, Norway) (Figure 1). TEST SUBSTANCES The antidiabetic drugs chlorpropamide, glibenclamide, glipizide, gliquidone, glymi- dine, tolazamide, and tolbutamide, and the diuretics bemetizide, bendroflumethiazide, benzylhydrochlorothiazide, bumetanide, butizide, chlortalidone, furosemide, hydro- chlorothiazide, hydroflumethiazide, indapamide, piretanide, polythiazide, trichlorme- thiazide, and xipamide were dissolved in dimethylsulphoxide to a final concentration of 0.25 M. When 1-10 }xl of these solutions were added to 5 ml PBS buffer and incubated together with the cells, final test substance concentrations ranged from 0.05-0.5 mmol/l. PHOTOTOXICITY ASSAY NHIK 3025 cells, derived from a human carcinoma in situ of the cervix (13) were subcultured twice a week in Medium E2a (14) (split ratio 1:100) containing 20% human serum and 10% horse serum. The cells, 400 per flask (25 ml), were left at 37øC for 24 hours before addition of the test substance and irradiation. The cells were incubated together with the test substances for one hour. In the antioxidant experiments, ascorbic acid, alpha-tocopheol, beta-carotene, or ubiquinone (Q 10) were added to the test suspensions. Ascorbic acid, alpha-tocopherol, and beta-carotene were purchased from Sigma Chemical Company. Ubiquinone (Q 10)
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)