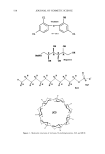
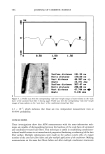
542 JOURNAL OF COSMETIC SCIENCE Keuls test, was performed on the mean inhibition zones and mean solubility values to identify significant differences in solubility and antibacterial activity (Statitica 5.1, Statsoft Inc., Tulsa, OK). P-values of less than 0.05 indicated significant differences in solubility. RESULTS AND DISCUSSION Triclosan (Figure 1) belongs to a class of compounds known as hydroxydiphenyl ethers. It is an anionic, lipophilic compound that is very poorly soluble in water. In this study, the solubility in water and pH 7.4 phosphate buffer was determined to be 0.002 mg/ml- • and 0.004 mg/ml- • at 30øC. In Figures 3 and 4 solubility profiles of triclosan in combination with increasing concentrations of three cyclodextrins and SLS are given. Previous researchers have reported the effect of cyclodextrins on the solubility of triclo- san (11,13-15). The results are given here as a basis for comparison, to evaluate the effect of other solubilizing agents on the solubility of triclosan. COMPLEXATION WITH CYCLODEXTRINS [•CD and HPI•CD both significantly increased the solubility of triclosan in water and the phosphate buffer (2000- to 4000-fold, Table I). Both cyclodextrins are soluble in water, but HPI•CD is more soluble because substitution of the hydroxyl groups of the [3CD disrupts the network of hydrogen bonding around the rim of the [•CD. As a result of disruption of the hydrogen-bonding network, the hydroxyl groups interact much more strongly with water, resulting in increased solubility compared to [3CD. Each 14 12 10 0.00 0.25 -•- Beta-CD J -e- Gamma-CD J -e-HPBCD • T 0.05 0.10 0.15 0.20 Concentration (M) Figure 3. Solubility of triclosan (mg/ml-•) at increasing concentrations of several cyclodextrins and SLS in water. The data points and error bars represent the mean and standard deviations of two replicates.
INCREASED AQUEOUS SOLUBILITY OF TRICLOSAN 543 12 & Beta.CD • +Gamma-CD ,-, 10 • HPBCD m 8 .= 6 o 4 2 T • T • T • T 0.00 0.05 0.10 0.15 0.20 0.25 Concentration (M) Figure 4. Solubility of triclosan (mg/ml-•) at increasing concentrations of several cyclodextrins and SLS in pH 7.4 phosphate buffer. The data points and error bars represent the mean and standard deviations of two replicates. Table I Maximum Solubility of Triclosan in Aqueous and Buffered Solubilizer Solutions With Corresponding Solubilizer Concentrations Water Phosphate buffer (pH 7.4) Triclosan* Triclosan Solubilizer Solubilizer (M) (mg/ml -•) Solubilizer (M) (mg/ml-•) N-methylglucamine 1.00'* 12.43 + 1.21 1.00'* 18.34 + 0.62 L-arginine 1.00'* 12.81 _+ 1.09 0.70 18.35 _+ 1.13 SLS 1.00'* 12.15 + 0.75 1.00'* 12.77 _+ 0.95 [3CB 0.15'* 8.11 + 0.79 0.18'* 7.89 + 0.81 HPIBCD 0.15 8.20 _+ 0.81 0.18 8.21 _+ 1.16 Ethanolamine 0.80 5.84 _+ 0.37 0.80 6.00 + 0.27 Sodium benzoate 0.80 5.95 -+ 0.40 0.80 2.20 + 0.16 Na-methylparaben 0.80 3.61 _+ 0.34 0.80 3.42 _+ 0.43 Triethanolamine 0.80 1.92 + 0.07 0.80 1.23 _+ 0.08 Diethanolamine 0.80 1.43 _+ 0.09 0.80 0.98 + 0.13 Glycine 0.80 0.22 _+ 0.01 0.80 0.32 _+ 0.01 •/CD 0.15 1.10 _+ 0.08 0.15 0.13 _+ 0.06 * Solubility of triclosan at 30øC was 0.002 mg/ml -• in water and 0.004 mg/ml -• in pH 7.4 buffer. ** Maximum solubility not reached. cyclodextrin molecule is composed of a ring of glucose molecules (Figure 1), which can accept a lipophilic guest such as triclosan within the ring. A variety of noncovalent forces, such as van der Waal forces, hydrophobic interaction, and dipole moment are responsible for formation of a stable complex. For triclosan, most probably the hydro- phobic portion of the guest interacts with the hydrophobic cavity of the cyclodextrin.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)