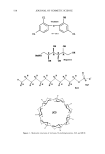
552 JOURNAL OF COSMETIC SCIENCE stabilize. It becomes brown in air due to oxidation. One gram of hydroquinone is soluble in 17 ml of water and freely soluble in alcohol. In antifreckle and hair dyeing cosmetic products, a law limits its content to 2% w/w (1). Most hydroquinone products used in creams, gels, lotions, and ointments are locally manufactured in Iran. Being located in a tropical area, some Iranian people suffer from solar effects on their skin. Freckles are one of these effects. Hydroquinone is one of the cheapest hypopig- menting agents available in the Iranian market, which most middle and lower class Iranian people have preferred to use along with sunscreening products. However, with low stability and the side effects of allergy and irritation from hydroquinone for many consumers, the Cosmetic Control Division of Iran has special concerns for these products. In 1995, the FDA and the Toxicology Division of the Department of Medical Sciences, Ministry of Health, in Thailand reported that six out of 20 samples collected from the market contained more hydroquinone than the allowed amount. This was excused because of the high instability of hydroquinone, and most manufacturers had put the excess hydroquinone in their products in order to maintain a constant amount of 2.0% during storage (1). Recently, many researchers have been involved in finding means to prevent or delay deterioration by oxidative reactions in cosmetic preparations. A variety of antioxidants, both from natural sources and synthetic processes, are available in the market. In Iran, antioxidants that are usually incorporated in hydroquinone formulations are sodium metabisulfite (SM), BHT (butylated hydroxy toluene), BHA (butylated hydroxy anisole), ascorbic acid, vitamin E, citric acid, and/or combinations of these chemicals. Their shelf life is about one year. Hydroquinone itself has been used as an antioxidant in combi- nation with others in the concentration of 0.05-0.1% (2). Various disadvantages of some of these antioxidants have caused concern. For example, an application of BHA and BHT is now restricted in many countries, since undesirable effects from these additives on the enzymes of the liver and lungs can occur. Occasionally, the antioxidant ability of vitamin E is less active (1). More recently, research has focused on developing safer and more effective antioxidants from natural sources, such as Rosmarin•s officina/is L., Piper spp., Geranium pratensis, Germ •rban•m, Viola tricolor, R•mex acetosa, Ilex parag•ensis, Rosa sp., licorice root, cinnamon, ginger rhizomes, Capsicum spp. and green tea (3-10). Some of the chemical constituents (e.g., polyphenolic flavonoids) of Glycyrrhiza glabra have been identified as antioxidant agents it is possible that the synergistic effects of flavonoid mixtures may be responsible for the high activity observed in licorice extract (6,11-16). However, a study of the antioxidative activity of the extract from Glycyrrhiza glabra has never been performed for cosmetic preparations. Glycyrrhiza glabra L. is good for skin eruptions, including dermatitis, eczema, pruritus, and cysts. It has also anti- inflammatory, anti-infecting, antiseptic, antibacterial, antihepatotoxic, antiviral, and antiphlogistic properties. It is also used for gastric and duodenal ulcer (17). Glycyrrhiza glabra L. is native to Eurasia and cultivated in Europe (Spain, Italy, France, etc), the Middle East (Syria, Iran, Turkey, Iraq, etc), and Asia (e.g., China). The parts used are the dried roots collected in the fall (18). Hydroquinone, which is known for its high sensitivity to oxidation, has been chosen as an indicator for comparison of the antioxi- dative activity of licorice extract to commercial antioxidants in the form of 2% w/w hydroquinone cream.
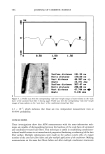
ANTIOXIDANT ACTIVITY OF LICORICE EXTRACT 553 MATERIALS AND METHODS EXTRACTION An amount of 250 g of the powdered dry roots of Glycyrrhiza glabra L. was soaked in 2500 ml of methanol (Merck, Germany) for 24 hrs. The mixture was filtered, and the tiltrate was evaporated to give a yield of 21.0% w/w of dried licorice extract. MATERIALS Cetyl alcohol, white petrolatum, mineral oil, Tween 80, NaOH, propylene glycol, butylated hydroxy toluene (BHT), and sodium metabisulfite (MS) were purchased from Merck (Germany). Methyl and propyl paraben were provided by Kech's (USA). Pemulen TR1 was received from the B.F. Goodrich Co. (USA). Deionized water was freshly prepared. PREPARATION OF TEST SAMPLES Hydroquinone cream was freshly prepared. The pemulen TR1 (2% w/w) was wetted in preserved water for 24 hr and dispersed with a double-bladed mixer (Ika-Werk, Ger- many) in 500 rpm for 10 min in a water bath at 75øC. Separately, cetyl alcohol (3% w/w), white petrolatum (8% w/w), mineral oil (8% w/w), and Tween 80 (1% w/w) were melted in a water bath at 70øC. The latter was added to the aqueous phase, and after neutralizing by NaOH solution (18% w/w) to pH 6.2, the mixture was stirred lOO .c: 90 '• 80 '• 70 ß • 60 ..-. 50 + 40 •: •o P 20 • '•o t,,. o C 25•0.5C I •45 + 0.5 C Figure 1. Comparison of the average percentage of hydroquinone remaining after incubation at 25 ø _+ 0.5øC and 45 ø _+ 0.5øC for three months. 1: CB (cream base) + HY (hydroquinone). 2: CB + HY + ext. (0.1%). 3: CB + HY + ext. (0.5%). 4: CB + HY + ext. (1%). 5: CB + HY + ext. (2%). 6: CB + HY + SM (0.1%). 7: CB + HY + SM (0.5%). 8: CB + HY + SM (1%). 9: CB + HY + SM (2%). 10: CB + HY + BHT (0.1%). 11: CB + HY + BHT (0.5%). 12: CB + HY + BHT (1%). 13: CB + HY + BHT (2%). i i i i , i i i i i i i i 0 I 2 3 4 5 6 7 8 9 10 11 12 13
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)