ANTIOXIDANT PHOTOPROTECTION 5 91 lightly pigmented, although, because of their ex vivo nature, their types were not classified. Sample 1 was obtained from the breast of one individual sample 2 was facial skin from a different individual. Age and gender are not specified due to the require- ments of the University of Illinois Internal Review Board. Samples were acquired immediately post-surgery and stored at 4øC in indicator-free RPMI media (Life Tech- nologies) supplemented with gentamicin and L-glutamine until use. Photoirradiation equipment. UV irradiation of each skin sample was achieved using two U¾ fluorescence bulbs (T120W/12RS, Philips). The lamp source peaked near 310 nm in the UVB before tapering into the UVA, where 20% of the photons came between 320 nm and 400 nm. Thus, the data acquired are predominately due to UYB-induced reactions. UV irradiance is determined using an energy meter (Model 1825-C, New- port). A total irradiance of 1600 J m -2 was selected for two reasons. First, the amount of DHR is not fully converted to rhodamine-123 at this irradiance, which indicates that the amount of DHR present is sufficient to react with the quantity of ROS by a dose of 1600 J m -2 (l). Second, an irradiance of 1600 J m -2 is a biologically significant U¾ dose and can be obtained from two hours of noonday summer solar UV exposure in the northern hemisphere (13). TWO-PHOTON FLUORESCENCE IMAGING MICROSCOPY AND ROS DETECTION IN SKIN Two-photon excitation is achieved when a molecule simultaneously absorbs two photons at the excitation wavelength. Typically, two-photon excitation is achieved using an ultrafast, pulsed, near-IR laser system (785 nm, 10 -•5 s, titanium:sapphire). Many are familiar with confocal microscopy, which is commercially available. However, compared to UV or visible confocal one-photon sources, two-photon excitation is advantageous for sectioned imaging of the skin, providing reduced photobleaching of the probe fiuoro- phore and limited photodamage to the sample. In addition, Masters et al. found that the absence of a pinhole in two-photon excitation allows for greater depth penetration into skin compared to UV confocal excitation (14). As a result, two-photon excitation pro- vides submicron spatial resolution with submillimeter depth penetration such that data can be acquired within the cells of each epidermal stratum and through to the upper derreal layers. In two-photon fluorescence intensity imaging microscopy, the sample is incubated with a fluorophore that can be excited at the two-photon excitation wave- length. Fluorescence probes for a number of chemical reactions or properties within biological samples are available, including those for ROS detection and pH within skin, where a change in fluorescence intensity or lifetime relative to a control sample yields information on the sample (1,15,16). For example, to study ROS photoprotection within human skin, a five-step procedure is followed to determine the effect of a topical formulation, like a sunscreen- or antioxi- dant-containing crbme, upon UV-induced ROS levels within ex vivo skin samples. First, approximately 2 mg cm -2 of the formulation is applied to the surface of a skin sample (-0.5 cmx 0.5 cm) and incubated for three hours at 4øC to maintain tissue viability. Second, the sample is incubated for ten minutes at room temperature in a solution containing the ROS-detecting probe dihydrorhodamine (DHR, 100 •M in 2:1 PBS/ EtOH). DHR is nonfluorescent until it reacts with •O2, H202, and/or ONOO- (and potentially other ROS), forming fluorescent rhodamine- 123 (R 123, emission maximum 525 nm) (Figure 1A). Third, the sample is imaged to obtain background (before UV) fluorescence levels for each epidermal stratum. Image areas are between 625 •m 2 and
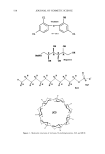
592 JOURNAL OF COSMETIC SCIENCE A non-fluorescent dihydrorhodam•e ROS •N (••NH•+ COOCH• fluorescent rhodamine-123 o o o II tl , •(CI•COCH•OCCH• (CH•OC•OCCH,• OCOCH•. i.•'.O. • .OCOCH• non-fluorescent calcein-am Esterase (COOH•)•NCH• CH•N(COOH0• HO i•o fluorescent calcein Figure 1. Molecular probes used to detect ROS (A) and esterase activity (B). 4000 pm 2 and are acquired ca. every 10 pm, beginning at the stratum corneum surface. Images in the z-direction as little as 2 pm apart can be acquired if desired. Fourth, the skin sample is irradiated by UV (T120W/12RS, Philips), and finally re-imaged. Each image acquired is composed of 256 x 256 pixels. A discussion of the UV source and its differences relative to solar UV radiation is out of the scope of this paper the reader is referred to other work (1). In our experiments two-photon excitation is achieved by a Nd:YVO 4 pumped (Millenia, Spectra-Physics) titanium:sapphire laser (Tsunami, Spectra-Physics), whose fundamental at 785 nm is coupled through the epifiuorescence port of a Zeiss Axiovert microscope. Fluorescence from the samples is collected by a photomultiplier tube (R3996, Hama- matsu). IMAGE ANALYSIS To determine the reduction in UV-induced ROS at depth z due to a test formulation, equation 1 is used: % - Reduction(z) = 100 - 100 (I(z) .... p/e• (1) • I(Z)control / At each epidermal depth z, the fluorescence intensity is calculated over the entire image. These intensity data are averaged together for each skin area studied (I(z)s•,e/). At least two unique areas are imaged per skin sample. I(Z)co,,ro • is calculated identically for each area, where the control images are those acquired on skin incubated with DHR only and indicate the control level of ROS that is generated at the UV irradiance used (1600 J m-2). The average reduction in ROS (% reduction•v a) is calculated by averaging all % reduction(z) values calculated from each tissue sample.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)