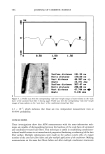
HAIR FIBER HYDRATION 531 DSC '101 Y(mV't, Ukng) ,•, •xo 0.14 /'•'•'-'•' • \ o.12 ////• o.io .,• / .._ 'xx'X • ,' / ,--"-" ..... --,. X / / ,-" "-._ •. X 0.08 / .• •" ',. 0.06 • . ' ' • •_,-' •ea: 273.5795 J• • / - '%,• ' '• ,..,• ---• • •• '• ............. "/ • ' ' "-• 0.• / .... • .... •_• [3] o s• • • •o T•r•re • Figure 3. Details of DSC curves for treated and untreated hair. Each curve is an example of the duplicate curves obtained. Bleached without treatment [1] ...... treatment 1 [2] , ß treatment 2 [3] .... DSC '101 J 0.14 0.12 0.10 0.04 Figure 4. Details of DSC curves for treated and untreated hair. Each curve is an example of the duplicate curves obtained. Without treatment [1] ...... treatment 3 [2] ß treatment 4 [3] , ß After the rinse-off treatments 3 and 4, these amounts of energy were, respectively, 17% and 41% higher than for the control. We suggest two hypotheses to explain the increased amounts of energy necessary for the release of water from hair. First, the treatments increase the water content in the fibers. The higher the amount of water attached to the fibers, the more energy will be necessary to release it. Second, the treatments would, in some way, hinder the release of water,
532 JOURNAL OF COSMETIC SCIENCE Table II Water Vaporization AH Values Obtained From the DSC Curves for Hair Subjected to Different Treatments Treatments AHvap, water (average)J/g Bleached, untreated hair 146 +_ 13 Treatment 1, not rinsed 210 +_ 5 Treatment 2, not rinsed 268 + 8 Untreated hair 164 +_ 19 Treatment 3, rinsed 192 + 7 Treatment 4, rinsed 231 + 2 which would thus require more energy. This interference may be due to the formation of a barrier (film) and/or to the presence of hydrophilic substances. Dias et al. (17) ascribe the retention of moisture in the hair to the formation of a silicone film. Cao (11) argues that the energy spent from 0øC to 200øC in the DSC analysis is associated with the release of water caused by heating the hair fibers. To ensure that the vapor released from heated hair contained only water, it was necessary to determine the chemical composition of the vapor because other substances, other than water, could volatilize at these temperatures. GC/TCD (gas chromatography/thermal conductivity detector) analysis of this vapor confirmed the presence of water and allowed its quantification. The results are shown in Figures 5 and 6. Water release for all the samples was observed to begin at 25øC and end at approximately 200øC. Since the GC detector identified nothing but water within this temperature range, the first section of the DSC curves corresponds only to the water present in the test samples. An increase in water content was observed above 200øC. This additional amount of water may result from the constitution of the hair fibers, as well as from the combustion of the entire organic material present in hair. $ [ • REFERENCE L •a- TREATMENT 2 / \ 4| = TREATMENT3 ! \ • • TREATMENT 4 0 0 50 1 O0 150 200 250 300 Reacion Temperature (ø-C) Figure 5. Water weight percentage observed in the vapor from the hair heat treatment.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)