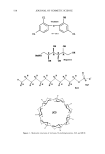
548 JOURNAL OF COSMETIC SCIENCE Table II Antimicrobial Activity, Measured by Zone Inhibition, of Triclosan and Solubilizing Agents Diameter of inhibition zone (mm) Compound E. coli P. aeruginosa S. aureus A. niger C. albicans Triclosan powder 18.8 No zone 19.5 17.4 14.5 N-methylglucamine 15.9 19.3 14.3 25.5 20.9 L-arginine No zone 14.7 No zone 24.6 20.1 Ethanolamine 23.9 24.6 12.9 27.8 18.4 SLS No zone No zone 18.2 28.6 15.1 [3CD No zone No zone No zone No zone No zone for mixtures of triclosan and the solubilizing agents prepared in water or the pH 7.4 phosphate buffer. In Table III the results for the phosphate buffer solutions are listed. The diameters of the zones of inhibition increased when a constant concentration of 6.0 mg/ml -• of triclosan was mixed with all the solubilizers. This showed that the bacte- riostatic efficacy of triclosan increased when combined with the solubilizers. Whether this was because of the bacteriostatic efficacy of the solubilizer, or a synergistic effect that occurred, is unknown. The results listed in Table III show that, on average, the combined solutions doubled the diameters of the inhibition zones. From these results, it is also clear that N- methylglucamine, L-arginine, and ethanolamine in combination with triclosan were the most effective antimicrobial combinations. Although not as effective as the other solu- bilizers, both [3CD and SLS also enhanced the growth inhibition of most of the organ- isms tested when combined with triclosan. The difference between the antimicrobial activity of solutions containing the complexing agents, N-methylglucamine, L-arginine, and ethanolamine, and the compounds that encapsulate the triclosan, [3CD and SLS, was significant. This difference could be the result of partial inactivation of the triclosan by miceliar entrapping on inclusion because these processes reduce the amount of free triclosan available in solution. Overall, triclosan/solubilizer combinations were most effective against A. niger and least effective against P. aeruginosa. CONCLUSIONS A solubilization study for triclosan in water and pH 7.4 phosphate buffer at 30 ø C Table III Antimicrobial Activity, Measured by Zone Inhibition, of Solutions Prepared in pH 7.4 Phosphate Buffer Containing Triclosan and Solubilizing Agents Diameter of inhibition zone (mm) Compound E. coli P. aeruginosa S. aureus A. niger C. albicans N-methylglucamine 34.2 17.4 32.7 42.4 29.4 L-arginine 34.9 19.1 34.1 44.2 31.4 Ethanolamine 35.0 23.5 31.5 42.5 31.2 SLS 23.5 12.4 21.0 30.4 23.2 •CD 30.5 No zone 23.1 27.8 27.2
INCREASED AQUEOUS SOLUBILITY OF TRICLOSAN 549 showed the following compounds as potential solubilizers of the drug. They are in order of their solubilizing performance: N-methylglucamine -- L-arginine SLS [3CD -- HP[3CD ethanolamine sodium benzoate sodium methyl 4-hydroxybenzoate triethanolamine -- diethanolamine. By using these solubilizers, the solubility of triclo- san was increased 80- to 6000-fold. The formation of either salts or complexes is postulated as a possible mechanism for the increase in the solubility of triclosan by these compounds. In addition, the combination of triclosan with some of these solubilizers increased the antimicrobial potency of triclosan. The mechanism for this increase in activity is unknown and needs further investigation. However, complexing and salt- forming agents improved the activity of triclosan significantly more than compounds that solubilized triclosan by miceliar or molecular inclusion. REFERENCES (1) (2) (3) (4) (5) (6) (7) (8) (9) (10) (11) (12) (13) (14) (15) (16) (17) C. A. Savage, A new bacteriostat for skin care products, Drug Cosmet. Ind., 109, 36-38, 163 (1971). H. P. Nissan and D. Ochs, Triclosan: An antimicrobial active ingredient with anti-inflammatory activity, Cosmet. Toiletr., 113, 61-64 (1998). V. Kjaerheim, P. Barkvoll, S.M. Waaler, and G. Roella, Triclosan inhibits histamine-induced in- flammation in human skin, J Clin. Periodont., 22, 423-426 (1995). R. D. Jones, H. B. Jampani, J. L. Newman, and A. S. Lee, Triclosan: A review of effectiveness and safety in health care settings, Am. J. Infect. Control., 28, 184-196 (2000). S. M. Waaler, G. Roella, K. K. Skjoerland, and B. Oegaard, Effects of oral rinsing with triclosan and sodium lauryl sulphate on dental plaque formation: A pilot study, Scand. J. Dent. Res., 101, 192-195 (1993). V. Kjaerheim, S. M. Waaler, and A. Kalvik, Experiments with two-phase plaque-inhibiting mouth- rinses, Eur. J. Oral Sci., 103, 179-181 (1995). L. M. McMurry, M. Oethinger, and S. B. Levy, Triclosan targets lipid synthesis, Nature, 394, 531-532 (1998). C. W. Levy, A. Roujeinikova, S. Sedelnikova, P. J. Baker, A. R. Stuitje, A. R. Slabas, D. W. Rice, and J. B. Rafferty, Molecular basis of triclosan activity, Nature, 398, 383-384 (1999). S. Namita and A. Surolia, Triclosan offers protection against blood stages of malaria by inhibiting enol-ACP reductase of Plasmodiumfalciparum, Nat. Med., 7, 167-173 (2001). R. Perozzo, M. Kuo, A. S. Sidhu, J. T. Valiyaveetti, R. Bittman, W. R. Jacobs, D. A. Fidock, and J. C. Sacchettini, Structural elucidation of the specificity of the antibacterial agent triclosan for malarial enoyl acyl carrier protein reductase, J. Biol. Chem., 277, 13106-13114 (2002). T. Loftsson, N. Leeves, N. Bjornsdottir, L. Duffy, and M. Masson, Effect ofcyclodextrins and polymers on triclosan availability and substantivity in toothpastes in vivo, J. Pharm. Sci., 88, 1254-1258 (1999). S. H. Yalkowsky, Ed., Techniques of Solubilization of Drugs (Marcel Dekker, New York, 1981), pp. 16-34, 91-134, 135-157. J. Lu, M. A. Hill, M. Hood, D. F. Greeson, J. R. Horton, P. E. Orndorff, A. S. Hemdon, and A. E. Tonelli, Formation of antibiotic, biodegradable polymers by processing with Irgasan DP 300R (tri- closan) and its inclusion compound with [3-cyclodextrin,J. Fiber Polym. Sci., 82, 300-309 (2001). P.M. Ashall, B. M. Kiernan, and N. R. Russell, Complexation of the bacteriostat triclosan the •-cy- clodextrin, Proceedings of the World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Tech- nology, Budapest, May 9-11, 1995, pp. 605-606. T. Loftsson, N. Leeves, J. F. Sigurjonsdottir, H.H. Sigurosson, and M. Masson, Sustained drug delivery system based on a cationic polymer and an anionic drug/cyclodextrin complex, Pharmazie, 56, 746-747 (2001). The United States Pharmacopeia, XXIVth Revision (United States Pharmacopeial Convention, Rockville, MD, 2000). J. K. Lim, H. O. Thompson, and C. Piantadosi, Solubilization and stability of phenobarbital by some aminoalcohols, J. Pharm. Sci., 53, 1161-1165 (1964).
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)