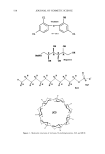
562 JOURNAL OF COSMETIC SCIENCE dynamically stable. In the cases of SCI and ACI, it is apparent that ACI has a lower T (below room temperature), since ACI has a very small lattice energy and SCI has a higher T (close to the boiling temperature of water) due to its large lattice energy. METHODS Three methods of preventing recrystallization of SCI have been developed based on the enthalpy of solubilization and equilibrium. The first focuses on secondary surfactants, which tie up CI- ions within micelies. The second focuses on the exchange of sodium ions with ammonium ions (and/or triethanolamonium). The third centers on introduc- ing emulsifiers and emollients to change micelies into stable emulsified oil drops. METHOD I: SELECTED SECONDARY SURFACTANTS When thinking about SCI solvation, it is important to refer to the equilibrium equation stated above. If one modifies an SCI/water system in such a way as to shift equilibrium to the right, away from solid SCI, then its solubility in water is increased. Modifying pure MIC(CI) with specific secondary surfactants will accomplish this goal both by increasing micelie stability and by increasing the number of aqueous CI- ions that are taken up within micelies. Generally, there are four types of interactions (or molecular interactions) involved in micelie formation (6). They are: (i) hydrophilic/hydrophobic interaction between sur- factant and water molecules (ii) interaction among solvated head groups (generally repulsive) and between the head groups and co-ions (iii) attractive interaction among hydrocarbon tails in separate molecules and (iv) geometric packing constraints derived from the particular molecular structure involved. The last two interactions are very important to the discussion of method I since interactive forces can vary significantly, depending on the surfactants. The second interaction listed will become a focus in method II. In order to tie CI- ions in micelies without recrystallization, one has to choose surfactants that strongly interact with CI- ions. To promote strong interaction with CI- ions, surfactants should have either similar hydrophilic head groups or larger and complicated hydrophilic head groups. The struc- ture of SCI is depicted in Figure 2 along with the structures of other selected surfactants. Anionic surfactants, amphoteric surfactants, and non-ionic surfactants can serve this purpose. In the anionic surfactant category, these are (but not limited to): sodium (or ammonium) dialkyl sulfosuccinates, disodium (or diammonium) alkyl sulfosuccinates, disodium (or diammonium) alkyl ether sulfosuccinates, disodium (or diammonium) alkylamido MEA sulfosuccinates, disodium (or diammonium) alkylamido MIPA sulfo- succinates, disodium (or diammonium) alkylamido PEG-2 sulfosuccinates, sodium (or ammonium) acyl taurates, disodium (or diammonium) acyl glutamates, sodium (or ammonium) acyl lactylates, and sodium acyl sarcosinate. In the amphoteric category (Figure 3) there are alkylamidopropyl hydroxysultaines, sodium (or ammonium) alkylamphoacetates, disodium (or diammonium) alkylampho- diacetates, sodium (or ammonium) alkylamphopropionates, disodium (or diammonium) alkyliminodipropionates, alkylamidopropyl betaines, alkylamidopropylamine oxides, and sodium alkylamphohydroxypropylsulfonate.
SOLUBILIZATION OF SCI 563 O RC--OCH2CH2SO3Na O NHCH2CH2SO3Na sodium cocoyl isethionate sodium acyl taurate NaOOCH•CH2CH2COONa NH--CR II o disodium acyl glutamate O O OCHC--OCHCOONa CH3 CH 3 sodium acyl lactylate O O RO(CH2CH20)x• CC H2¾H C-- O Na SO3Na sodium alkyl ether sulfosuceinate 0 0 RO•CCH2t•HC--O SO3Na sodium dialkyl sulfosuccinate RO 0 0 CCH2(•HC--ONa SO3Na O NCH2COONa I CH3 disodium alkyl sulfosuccinate sodium acyl sarcosinate Figure 2. Structures of selected anionic surfactants. Non-ionic surfactants require complicated and long-chain polyethylene groups to pre- vent recrystallization of SCI. These surfactants (Figure 4) are polysorbate 20, polysorbate 60, polysorbate 80, alkyl glucosides, PEG-n (n -- 20-80) glyceryl stearates, PEG-n (n = 20-80) glyceryl isostearates, PEG-20-PPG-10 glyceryl stearates, PEG-n (n = 20-80) glyceryl oleates, PEG-n (n = 20-80) glyceryl cocoates, and PEG-n (n: 20-80) glyceryl laurates. Other non-ionic surfactants are less effective in this application. It should be emphasized that micelie stability is an essential element in shifting the SCI solubiliza- tion equilibrium to the right, thus increasing solubility. When a surfactant system consists of head groups that are similar to or larger and more complicated than those of SCI, micelle stability will increase and keep SCI dissolved.
Purchased for the exclusive use of nofirst nolast (unknown) From: SCC Media Library & Resource Center (library.scconline.org)